HBZ stimulates brain-derived neurotrophic factor/TrkB autocrine/paracrine signaling to promote survival of human T-cell leukemia virus type 1-Infected T cells
- PMID: 25210182
- PMCID: PMC4249074
- DOI: 10.1128/JVI.02285-14
HBZ stimulates brain-derived neurotrophic factor/TrkB autocrine/paracrine signaling to promote survival of human T-cell leukemia virus type 1-Infected T cells
Abstract
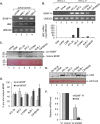
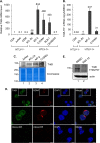
Brain-derived neurotrophic factor (BDNF) is a neurotrophin that promotes neuronal proliferation, survival, and plasticity. These effects occur through autocrine and paracrine signaling events initiated by interactions between secreted BDNF and its high-affinity receptor, TrkB. A BDNF/TrkB autocrine/paracrine signaling loop has additionally been implicated in augmenting the survival of cells representing several human cancers and is associated with poor patient prognosis. Adult T-cell leukemia (ATL) is a fatal malignancy caused by infection with the complex retrovirus human T-cell leukemia virus type 1 (HTLV-1). In this study, we found that the HTLV-1-encoded protein HBZ activates expression of BDNF, and consistent with this effect, BDNF expression is elevated in HTLV-1-infected T-cell lines compared to uninfected T cells. Expression of TrkB is also higher in HTLV-1-infected T-cell lines than in uninfected T cells. Furthermore, levels of both BDNF and TrkB mRNAs are elevated in peripheral blood mononuclear cells (PBMCs) from ATL patients, and ATL patient sera contain higher concentrations of BDNF than sera from noninfected individuals. Finally, chemical inhibition of TrkB signaling increases apoptosis in HTLV-1-infected T cells and reduces phosphorylation of glycogen synthase kinase 3β (GSK-3β), a downstream target in the signaling pathway. These results suggest that HBZ contributes to an active BDNF/TrkB autocrine/paracrine signaling loop in HTLV-1-infected T cells that enhances the survival of these cells.
Importance: Infection with human T-cell leukemia virus type 1 (HTLV-1) can cause a rare form of leukemia designated adult T-cell leukemia (ATL). Because ATL patients are unresponsive to chemotherapy, this malignancy is fatal. As a retrovirus, HTLV-1 integrates its genome into a host cell chromosome in order to utilize host factors for replication and expression of viral proteins. However, in infected cells from ATL patients, the viral genome is frequently modified to block expression of all but a single viral protein. This protein, known as HBZ, is therefore believed to modulate cellular pathways necessary for the leukemic state and the chemotherapeutic resistance of the cell. Here we provide evidence to support this hypothesis. We found that HBZ promotes a BDNF/TrkB autocrine/paracrine signaling pathway that is known to enhance the survival and chemotherapeutic resistance of other types of cancer cells. It is possible that inhibition of this pathway may improve treatments for ATL.
Copyright © 2014, American Society for Microbiology. All Rights Reserved.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

