In vitro activities of amphotericin B deoxycholate and liposomal amphotericin B against 604 clinical yeast isolates
- PMID: 25210203
- PMCID: PMC4250836
- DOI: 10.1099/jmm.0.075507-0
In vitro activities of amphotericin B deoxycholate and liposomal amphotericin B against 604 clinical yeast isolates
Abstract
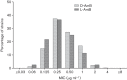
We determined the in vitro antifungal activity of liposomal amphotericin B (L-AmB) against 604 clinical yeast isolates. Amphotericin B deoxycholate (D-AmB) was tested in parallel against all the isolates. Susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) M27-A3 method. Overall, L-AmB was highly active against the isolates (mean MIC, 0.42 µg ml(-1); MIC90, 1 µg ml(-1); 97.2 % of MICs were ≤1 µg ml(-1)) and comparable to D-AmB (mean MIC, 0.48 µg ml(-1); MIC90, 1 µg ml(-1); 97.3 % of MICs were ≤1 µg ml(-1)). The in vitro activity of D-AmB and L-AmB was correlated (R(2) = 0.61; exp(b), 2.3; 95 % CI, 2.19-2.44, P<0.001). Candida albicans (mean MICs of D-AmB and L-AmB, 0.39 µg ml(-1) and 0.31 µg ml(-1), respectively) and Candida parapsilosis (mean MICs of D-AmB and L-AmB, 0.38 µg ml(-1) and 0.35 µg ml(-1), respectively) were the species most susceptible to the agents tested, while Candida krusei (currently named Issatchenkia orientalis) (mean MICs of D-AmB and L-AmB, 1.27 µg ml(-1) and 1.13 µg ml(-1), respectively) was the least susceptible. The excellent in vitro activity of L-AmB may have important implications for empirical treatment approaches and support its role in treatment of a wide range of invasive infections due to yeasts.
© 2014 The Authors.
Figures
References
-
- Carrillo-Muñoz A. J., Quindós G., Tur C., Ruesga M. T., Miranda Y., del Valle O., Cossum P. A., Wallace T. L. (1999). In-vitro antifungal activity of liposomal nystatin in comparison with nystatin, amphotericin B cholesteryl sulphate, liposomal amphotericin B, amphotericin B lipid complex, amphotericin B desoxycholate, fluconazole and itraconazole. J Antimicrob Chemother 44, 397–401. 10.1093/jac/44.3.397 - DOI - PubMed
-
- Cifani C., Costantino S., Massi M., Berrino L. (2012). Commercially available lipid formulations of amphotericin B: are they bioequivalent and therapeutically equivalent? Acta Biomed 83, 154–163. - PubMed
-
- CLSI (2008). Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts; Approved standard, 3rd edn, M27-A3. Wayne, PA: Clinical and Laboratory Standards Institute.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical