α-Lipoic acid inhibits Helicobacter pylori-induced oncogene expression and hyperproliferation by suppressing the activation of NADPH oxidase in gastric epithelial cells
- PMID: 25210229
- PMCID: PMC4152957
- DOI: 10.1155/2014/380830
α-Lipoic acid inhibits Helicobacter pylori-induced oncogene expression and hyperproliferation by suppressing the activation of NADPH oxidase in gastric epithelial cells
Abstract
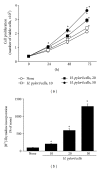
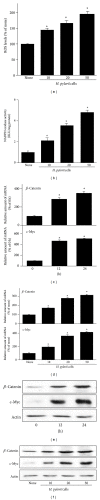
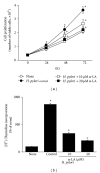
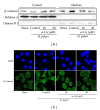
Hyperproliferation and oncogene expression are observed in the mucosa of Helicobacter pylori- (H. pylori-) infected patients with gastritis or adenocarcinoma. Expression of oncogenes such as β-catenin and c-myc is related to oxidative stress. α-Lipoic acid (α-LA), a naturally occurring thiol compound, acts as an antioxidant and has an anticancer effect. The aim of this study is to investigate the effect of α-LA on H. pylori-induced hyperproliferation and oncogene expression in gastric epithelial AGS cells by determining cell proliferation (viable cell numbers, thymidine incorporation), levels of reactive oxygen species (ROS), NADPH oxidase activation (enzyme activity, subcellular levels of NADPH oxidase subunits), activation of redox-sensitive transcription factors (NF-κB, AP-1), expression of oncogenes (β-catenin, c-myc), and nuclear localization of β-catenin. Furthermore, we examined whether NADPH oxidase mediates oncogene expression and hyperproliferation in H. pylori-infected AGS cells using treatment of diphenyleneiodonium (DPI), an inhibitor of NADPH oxidase. As a result, α-LA inhibited the activation of NADPH oxidase and, thus, reduced ROS production, resulting in inhibition on activation of NF-κB and AP-1, induction of oncogenes, nuclear translocation of β-catenin, and hyperproliferation in H. pylori-infected AGS cells. DPI inhibited H. pylori-induced activation of NF-κB and AP-1, oncogene expression and hyperproliferation by reducing ROS levels in AGS cells. In conclusion, we propose that inhibiting NADPH oxidase by α-LA could prevent oncogene expression and hyperproliferation occurring in H. pylori-infected gastric epithelial cells.
Figures




Similar articles
-
α-Lipoic Acid Inhibits Expression of IL-8 by Suppressing Activation of MAPK, Jak/Stat, and NF-κB in H. pylori-Infected Gastric Epithelial AGS Cells.Yonsei Med J. 2016 Jan;57(1):260-4. doi: 10.3349/ymj.2016.57.1.260. Yonsei Med J. 2016. PMID: 26632410 Free PMC article.
-
Activation of NF-κB and AP-1 Mediates Hyperproliferation by Inducing β-Catenin and c-Myc in Helicobacter pylori-Infected Gastric Epithelial Cells.Yonsei Med J. 2016 May;57(3):647-51. doi: 10.3349/ymj.2016.57.3.647. Yonsei Med J. 2016. PMID: 26996564 Free PMC article.
-
Lycopene treatment inhibits activation of Jak1/Stat3 and Wnt/β-catenin signaling and attenuates hyperproliferation in gastric epithelial cells.Nutr Res. 2019 Oct;70:70-81. doi: 10.1016/j.nutres.2018.07.010. Epub 2018 Jul 21. Nutr Res. 2019. PMID: 30098838
-
Diphenyleneiodonium Inhibits Apoptotic Cell Death of Gastric Epithelial Cells Infected with Helicobacter pylori in a Korean Isolate.Yonsei Med J. 2015 Jul;56(4):1150-4. doi: 10.3349/ymj.2015.56.4.1150. Yonsei Med J. 2015. PMID: 26069142 Free PMC article.
-
Polyamine- and NADPH-dependent generation of ROS during Helicobacter pylori infection: A blessing in disguise.Free Radic Biol Med. 2017 Apr;105:16-27. doi: 10.1016/j.freeradbiomed.2016.09.024. Epub 2016 Sep 25. Free Radic Biol Med. 2017. PMID: 27682363 Free PMC article. Review.
Cited by
-
Alpha-lipoic acid prevents atrial electrical and structural remodeling via inhibition of NADPH oxidase in a rabbit rapid atrial pacing model.Turk J Med Sci. 2022 Aug;52(4):1378-1388. doi: 10.55730/1300-0144.5445. Epub 2022 Aug 10. Turk J Med Sci. 2022. PMID: 36326363 Free PMC article.
-
α-Lipoic Acid Inhibits Expression of IL-8 by Suppressing Activation of MAPK, Jak/Stat, and NF-κB in H. pylori-Infected Gastric Epithelial AGS Cells.Yonsei Med J. 2016 Jan;57(1):260-4. doi: 10.3349/ymj.2016.57.1.260. Yonsei Med J. 2016. PMID: 26632410 Free PMC article.
-
α-Lipoic Acid Inhibits IL-8 Expression by Activating Nrf2 Signaling in Helicobacter pylori-infected Gastric Epithelial Cells.Nutrients. 2019 Oct 19;11(10):2524. doi: 10.3390/nu11102524. Nutrients. 2019. PMID: 31635029 Free PMC article.
-
Phycocyanin inhibits Helicobacter pylori-induced hyper-proliferation in AGS cells via activation of the ROS/MAPK signaling pathway.Ann Transl Med. 2022 Feb;10(4):176. doi: 10.21037/atm-21-7045. Ann Transl Med. 2022. PMID: 35280408 Free PMC article.
-
Inhibitory Effect of β-Carotene on Helicobacter pylori-Induced TRAF Expression and Hyper-Proliferation in Gastric Epithelial Cells.Antioxidants (Basel). 2019 Dec 11;8(12):637. doi: 10.3390/antiox8120637. Antioxidants (Basel). 2019. PMID: 31835889 Free PMC article.
References
-
- Parsonnet J, Friedman GD, Vandersteen DP, et al. Helicobacter pylori infection and the risk of gastric carcinoma. The New England Journal of Medicine. 1991;325(16):1127–1131. - PubMed
-
- Nomura A, Stemmermann GN, Chyou P, Kato I, Perez-Perez GI, Blaser MJ. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. The New England Journal of Medicine. 1991;325(16):1132–1136. - PubMed
-
- Uemura N, Okamoto S, Yamamoto S, et al. Helicobacter pylori infection and the development of gastric cancer. The New England Journal of Medicine. 2001;345(11):784–789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

