Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema
- PMID: 25210429
- PMCID: PMC4155807
- DOI: 10.2147/OPTH.S60893
Clinical utilization of anti-vascular endothelial growth-factor agents and patient monitoring in retinal vein occlusion and diabetic macular edema
Abstract
Purpose: To examine the utilization of bevacizumab and ranibizumab and disease monitoring in patients with branch or central retinal vein occlusion (BRVO/CRVO) or diabetic macular edema (DME) in clinical practice.
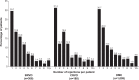
Patients and methods: This retrospective claims analysis included newly diagnosed patients with one or more bevacizumab or ranibizumab injections. Bevacizumab or ranibizumab utilization was assessed by year of first injection: 2008-2010 cohorts (12-month follow-up), January to June 2011 cohort (6-month follow-up). The main outcome measures were mean annual numbers of injections, ophthalmologist visits and optical coherence tomography examinations, and proportion of patients with additional laser or intravitreal triamcinolone (IVTA) use.
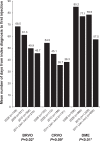
Results: A total of 885 BRVO, 611 CRVO, and 2,733 DME patients treated with bevacizumab were included, with too few ranibizumab-treated patients for meaningful analysis. Across the 2008, 2009, and 2010 cohorts, mean annual numbers of bevacizumab injections increased, but remained low (BRVO 2.5, 3.1, 3.3; CRVO 3.1, 3.1, 3.5; and DME 2.2, 2.5, 3.6, respectively); mean ophthalmologist visits ranged between 4.4 and 6.5, and mean optical coherence tomography examinations ranged between 3.1 and 3.9 across all conditions. A total of 42.0% of BRVO, 16.5% of CRVO, and 57.7% of DME patients received additional laser or IVTA therapy. The number of bevacizumab injections was positively associated with laser use in BRVO (3.3 versus 2.9, P<0.03), and with laser or IVTA use in DME (laser, 3.3 versus 2.7, P<0.03; IVTA, 3.3 versus 3.0, P<0.05).
Conclusion: During the study period (2008-2011), bevacizumab was the main anti-VEGF therapy used in clinical practice for BRVO, CRVO, and DME. Patients treated with bevacizumab were monitored less frequently and received fewer injections than patients in major clinical trials of ranibizumab.
Keywords: anti-vascular endothelial growth factor; bevacizumab; diabetic macular edema; intravitreal; ranibizumab; retinal vein occlusion.
Figures




References
-
- Yau JW, Lee P, Wong TY, Best J, Jenkins A. Retinal vein occlusion: an approach to diagnosis, systemic risk factors and management. Intern Med J. 2008;38(12):904–910. - PubMed
-
- Turello M, Pasca S, Daminato R, et al. Retinal vein occlusion: evaluation of “classic” and “emerging” risk factors and treatment. J Thromb Thrombolysis. 2010;29(4):459–464. - PubMed
-
- Institute for Clinical and Economic Review (ICER) Technology Assessment Report: Anti-Vascular Endothelial Growth Factor Treatment for Diabetic Macular Edema. Boston: ICER; 2012. [Accessed June 10, 2013]. Available from: http://www.cms.gov/Medicare/Coverage/DeterminationProcess/downloads/id85....
LinkOut - more resources
Full Text Sources
Other Literature Sources

