Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns
- PMID: 25210450
- PMCID: PMC4154894
- DOI: 10.2147/COPD.S62750
Management of COPD in the UK primary-care setting: an analysis of real-life prescribing patterns
Abstract
Background: Despite the availability of national and international guidelines, evidence suggests that chronic obstructive pulmonary disease (COPD) treatment is not always prescribed according to recommendations. This study evaluated the current management of patients with COPD using a large UK primary-care database.
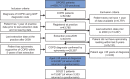
Methods: This analysis used electronic patient records and patient-completed questionnaires from the Optimum Patient Care Research Database. Data on current management were analyzed by the Global Initiative for Chronic Obstructive Lung Disease (GOLD) group and presence or absence of a concomitant asthma diagnosis, in patients with a COPD diagnosis at ≥35 years of age and with spirometry results supportive of the COPD diagnosis.
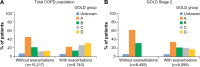
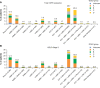
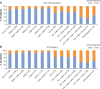
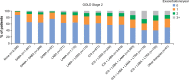
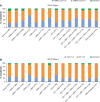
Results: A total of 24,957 patients were analyzed, of whom 13,557 (54.3%) had moderate airflow limitation (GOLD Stage 2 COPD). The proportion of patients not receiving pharmacologic treatment for COPD was 17.0% in the total COPD population and 17.7% in the GOLD Stage 2 subset. Approximately 50% of patients in both cohorts were receiving inhaled corticosteroids (ICS), either in combination with a long-acting β2-agonist (LABA; 26.7% for both cohorts) or a LABA and a long-acting muscarinic antagonist (LAMA; 23.2% and 19.9%, respectively). ICS + LABA and ICS + LABA + LAMA were the most frequently used treatments in GOLD Groups A and B. Of patients without concomitant asthma, 53.7% of the total COPD population and 50.2% of the GOLD Stage 2 subset were receiving ICS. Of patients with GOLD Stage 2 COPD and no exacerbations in the previous year, 49% were prescribed ICS. A high proportion of GOLD Stage 2 COPD patients were symptomatic on their current management (36.6% with modified Medical Research Council score ≥2; 76.4% with COPD Assessment Test score ≥10).
Conclusion: COPD is not treated according to GOLD and National Institute for Health and Care Excellence recommendations in the UK primary-care setting. Some patients receive no treatment despite experiencing symptoms. Among those on treatment, most receive ICS irrespective of severity of airflow limitation, asthma diagnosis, and exacerbation history. Many patients on treatment continue to have symptoms.
Keywords: COPD; UK primary-care setting; bronchodilators; inhaled corticosteroids; prescribing patterns.
Figures
References
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD 2014) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: Updated 2014. Global Initiative for Chronic Obstructive Lung Disease; 2014. [Accessed June 18, 2014]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014.pdf.
-
- Jones PW, Harding G, Berry P, Wiklund I, Chen WH, Kline Leidy N. Development and first validation of the COPD Assessment Test. Eur Respir J. 2009;34(3):648–654. - PubMed
-
- Global Initiative for Chronic Obstructive Lung Disease (GOLD 2014) Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease: Updated 2014. Global Initiative for Chronic Obstructive Lung Disease; 2014. [Accessed June 18, 2014]. Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2014.pdf.
-
- National Institute for Health and Care Excellence . NICE Guidelines [CG101]: Chronic Obstructive Pulmonary Disease: Management of Chronic Obstructive Pulmonary Disease in Adults in Primary and Secondary Care (Partial Update) London: National Institute for Health and Care Excellence; 2010. [Accessed June 18, 2014]. Available from: http://www.nice.org.uk/guidance/CG101.
-
- Cazzola M, Matera MG. Long-acting bronchodilators are the first-choice option for the treatment of stable COPD. Chest. 2004;125(1):9–11. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

