Molecular Differences and Similarities Between Alzheimer's Disease and the 5XFAD Transgenic Mouse Model of Amyloidosis
- PMID: 25210460
- PMCID: PMC4154482
- DOI: 10.4137/BCI.S13025
Molecular Differences and Similarities Between Alzheimer's Disease and the 5XFAD Transgenic Mouse Model of Amyloidosis
Abstract
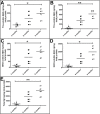
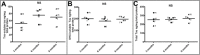
Transgenic (Tg) mouse models of Alzheimer's disease (AD) have been extensively used to study the pathophysiology of this dementia and to test the efficacy of drugs to treat AD. The 5XFAD Tg mouse, which contains two presenilin-1 and three amyloid precursor protein (APP) mutations, was designed to rapidly recapitulate a portion of the pathologic alterations present in human AD. APP and its proteolytic peptides, as well as apolipoprotein E and endogenous mouse tau, were investigated in the 5XFAD mice at 3 months, 6 months, and 9 months. AD and nondemented subjects were used as a frame of reference. APP, amyloid-beta (Aβ) peptides, APP C-terminal fragments (CT99, CT83, AICD), β-site APP-cleaving enzyme, and APLP1 substantially increased with age in the brains of 5XFAD mice. Endogenous mouse tau did not show age-related differences. The rapid synthesis of Aβ and its impact on neuronal loss and neuroinflammation make the 5XFAD mice a desirable paradigm to model AD.
Keywords: AICD; Alzheimer’s disease; BACE1; amyloid precursor protein; presenilin; tau; transgenic mice.
Figures

Similar articles
-
A combination Alzheimer's therapy targeting BACE1 and neprilysin in 5XFAD transgenic mice.Mol Brain. 2015 Mar 25;8:19. doi: 10.1186/s13041-015-0110-5. Mol Brain. 2015. PMID: 25884928 Free PMC article.
-
Effects of BACE1 haploinsufficiency on APP processing and Aβ concentrations in male and female 5XFAD Alzheimer mice at different disease stages.Neuroscience. 2015 Oct 29;307:128-37. doi: 10.1016/j.neuroscience.2015.08.037. Epub 2015 Aug 24. Neuroscience. 2015. PMID: 26314636 Free PMC article.
-
Mechanisms underlying insulin deficiency-induced acceleration of β-amyloidosis in a mouse model of Alzheimer's disease.PLoS One. 2012;7(3):e32792. doi: 10.1371/journal.pone.0032792. Epub 2012 Mar 5. PLoS One. 2012. PMID: 22403710 Free PMC article.
-
Modeling Alzheimer's disease in transgenic mice: effect of age and of presenilin1 on amyloid biochemistry and pathology in APP/London mice.Exp Gerontol. 2000 Sep;35(6-7):831-41. doi: 10.1016/s0531-5565(00)00149-2. Exp Gerontol. 2000. PMID: 11053674 Review.
-
Alzheimer's disease.Subcell Biochem. 2012;65:329-52. doi: 10.1007/978-94-007-5416-4_14. Subcell Biochem. 2012. PMID: 23225010 Review.
Cited by
-
Contributions of sex and genotype to exploratory behavior differences in an aged humanized APOE mouse model of late-onset Alzheimer's disease.Learn Mem. 2022 Sep 2;29(9):321-331. doi: 10.1101/lm.053588.122. Print 2022 Sep. Learn Mem. 2022. PMID: 36206387 Free PMC article.
-
Alzheimer's Disease as a Membrane Dysfunction Tauopathy? New Insights into the Amyloid Cascade Hypothesis.Int J Mol Sci. 2024 Sep 7;25(17):9689. doi: 10.3390/ijms25179689. Int J Mol Sci. 2024. PMID: 39273636 Free PMC article. Review.
-
Omega-3 Fatty Acid-Type Docosahexaenoic Acid Protects against Aβ-Mediated Mitochondrial Deficits and Pathomechanisms in Alzheimer's Disease-Related Animal Model.Int J Mol Sci. 2020 May 29;21(11):3879. doi: 10.3390/ijms21113879. Int J Mol Sci. 2020. PMID: 32486013 Free PMC article.
-
Synaptosomal Mitochondrial Dysfunction in 5xFAD Mouse Model of Alzheimer's Disease.PLoS One. 2016 Mar 4;11(3):e0150441. doi: 10.1371/journal.pone.0150441. eCollection 2016. PLoS One. 2016. PMID: 26942905 Free PMC article.
-
A Negative Energy Balance Is Associated with Metabolic Dysfunctions in the Hypothalamus of a Humanized Preclinical Model of Alzheimer's Disease, the 5XFAD Mouse.Int J Mol Sci. 2021 May 20;22(10):5365. doi: 10.3390/ijms22105365. Int J Mol Sci. 2021. PMID: 34065168 Free PMC article.
References
-
- Müller UC, Pietrzik CU, Deller T. The physiological functions of the β-amyloid precursor protein APP. Exp Brain Res. 2012;217:3–4. 325–329. - PubMed
-
- Van Nostrand WE, Schmaier AH, Farrow JS, Cunningham DD. Platelet protease nexin-2/amyloid beta-protein precursor. Possible pathologic and physiologic functions. Ann N Y Acad Sci. 1991;640:140–144. - PubMed
-
- Mahdi F, Van Nostrand WE, Schmaier AH. Protease nexin-2/amyloid beta-protein precursor inhibits factor Xa in the prothrombinase complex. J Biol Chem. 1995;270(40):23468–23474. - PubMed
-
- Henry A, Li QX, Galatis D, et al. Inhibition of platelet activation by the Alzheimer’s disease amyloid precursor protein. Br J Haematol. 1998;103(2):402–415. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

