ER-α36: a novel biomarker and potential therapeutic target in breast cancer
- PMID: 25210466
- PMCID: PMC4155893
- DOI: 10.2147/OTT.S65345
ER-α36: a novel biomarker and potential therapeutic target in breast cancer
Abstract
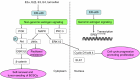
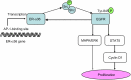
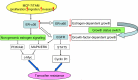
Estrogen receptor-alpha36 (ER-α36) is a 36-kDa variant of estrogen receptor-alpha (ER-α) firstly identified and cloned by Wang et al in 2005. It lacks both transactivation domains (activation function 1 and activation function 2) and has different biological characteristics compared to traditional ER-α (ER-α66). ER-α36 primarily locates on plasma membrane and cytoplasm and functions as a mediator in the rapid membrane-initiated non-genomic signaling pathway. Additionally, it inhibits the traditional genomic signaling pathway mediated by ER-α66 in a dominant-negative pattern. Accumulating evidence has demonstrated that ER-α36 regulates the physiological function of various tissues. Thus, dysregulation of ER-α36 is closely associated with plenty of diseases including cancers. ER-α36 is recognized as a molecular abnormality which solidly correlates to carcinogenesis, aggressiveness, and therapeutic response of breast cancer. Additionally, special attention has been paid to the role of ER-α36 in endocrine therapy resistance. Therefore, ER-α36 provides a novel biomarker of great value for diagnosis, prognosis, and treatment of breast cancer. It may also be a potential therapeutic target for breast cancer patients, especially for those who are resistant to endocrine therapy. In this review, we will overview and update the biological characteristics, underlying mechanism, and function of ER-α36, focusing on its biological function in breast cancer and endocrine therapy resistance. We will evaluate its application value in clinical practice.
Keywords: ER; ER-α36; breast cancer; endocrine therapy resistance.
Figures

References
-
- Nilsson S, Mäkelä S, Treuter E, et al. Mechanisms of estrogen action. Physiol Rev. 2001;81(4):1535–1565. - PubMed
-
- Weihua Z, Andersson S, Cheng G, Simpson ER, Warner M, Gustafsson JA. Update on estrogen signaling. FEBS Lett. 2003;546(1):17–24. - PubMed
-
- Kong EH, Pike AC, Hubbard RE. Structure and mechanism of the oestrogen receptor. Biochem Soc Trans. 2003;31(Pt 1):56–59. - PubMed
-
- Gustafsson JA. Estrogen receptor beta – a new dimension in estrogen mechanism of action. J Endocrinol. 1999;163(3):379–383. - PubMed
-
- Greene GL, Gilna P, Waterfield M, Baker A, Hort Y, Shine J. Sequence and expression of human estrogen receptor complementary DNA. Science. 1986;231(4742):1150–1154. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

