The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice
- PMID: 25210499
- PMCID: PMC4159692
- DOI: 10.7150/ijbs.9997
The prostate basal cell (BC) heterogeneity and the p63-positive BC differentiation spectrum in mice
Abstract
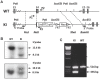
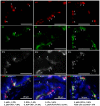
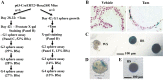
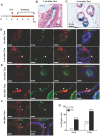
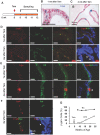
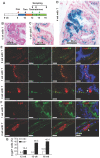
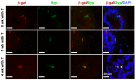
The prostate epithelium is composed of basal (BC), luminal (LEC), and neuroendocrine (NEC) cells. It is unclear how many subtypes of BCs in the prostate and which subtype of BCs contains the main stem cell niche in the adult prostate. Here we report seven BC subpopulations according to their p63, cytokeratin 14 (K14) and K5 expression patterns, including p63-positive/K14-negative/K5-negative (p63+/K14-/K5-), p63-/K14+/K5-, p63-/K14-/K5+, p63+/K14+/K5-, p63+/K14-/K5+, p63-/K14+/K5+, and p63+/K14+/K5+ BCs. We generated a p63-CreERT2 knock-in mouse line that expresses tamoxifen-inducible Cre activity in the p63-expressing cells, including the prostate BCs. We then crossbred this line with ROSA26R mice, and generated p63-CreERT2×ROSA26R bi-genic mice harboring the Cre-activated β-galactosidase reporter gene. We treated these bi-genic mice with tamoxifen to mark the p63+ BCs at different ages or under different hormonal conditions, and then traced the lineage differentiation of these genetically labeled BCs. We discovered that these p63+ BCs contain self-renewable stem cells in culture and efficiently differentiated into LECs, NECs and BCs in the postnatal, adult and re-generating mouse prostates. Therefore, BC population contains heterogeneous BCs that express different combinations of the p63, K14 and K5 differentiation markers. Because K14+ and K5+ BCs were previously shown to be extremely inefficient to produce LECs in adulthood, we propose that the p63+/K5-/K14- subpopulation of BCs contains most stem-like cells, especially in adult animals.
Keywords: Prostate epithelium; basal cell differentiation; lineage tracing; p63; stem cell..
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures
Similar articles
-
p63-expressing cells are the stem cells of developing prostate, bladder, and colorectal epithelia.Proc Natl Acad Sci U S A. 2013 May 14;110(20):8105-10. doi: 10.1073/pnas.1221216110. Epub 2013 Apr 25. Proc Natl Acad Sci U S A. 2013. PMID: 23620512 Free PMC article.
-
An active role of the DeltaN isoform of p63 in regulating basal keratin genes K5 and K14 and directing epidermal cell fate.PLoS One. 2009 May 20;4(5):e5623. doi: 10.1371/journal.pone.0005623. PLoS One. 2009. PMID: 19461998 Free PMC article.
-
Human tracheobronchial basal cells. Normal versus remodeling/repairing phenotypes in vivo and in vitro.Am J Respir Cell Mol Biol. 2013 Dec;49(6):1127-34. doi: 10.1165/rcmb.2013-0049OC. Am J Respir Cell Mol Biol. 2013. PMID: 23927678 Free PMC article.
-
Cellular and molecular biology of the prostate: stem cell biology.Urology. 2003 Nov;62(5 Suppl 1):11-20. doi: 10.1016/s0090-4295(03)00758-1. Urology. 2003. PMID: 14607213 Review.
-
Epithelial cell differentiation in the human prostate epithelium: implications for the pathogenesis and therapy of prostate cancer.Crit Rev Oncol Hematol. 2003 Jun 27;46 Suppl:S3-10. doi: 10.1016/s1040-8428(03)00059-3. Crit Rev Oncol Hematol. 2003. PMID: 12850522 Review.
Cited by
-
Knockout of SRC-1 and SRC-3 in Mice Decreases Cardiomyocyte Proliferation and Causes a Noncompaction Cardiomyopathy Phenotype.Int J Biol Sci. 2015 Jul 15;11(9):1056-72. doi: 10.7150/ijbs.12408. eCollection 2015. Int J Biol Sci. 2015. PMID: 26221073 Free PMC article.
-
Stem cells in genetically-engineered mouse models of prostate cancer.Endocr Relat Cancer. 2015 Dec;22(6):T199-208. doi: 10.1530/ERC-15-0367. Epub 2015 Sep 4. Endocr Relat Cancer. 2015. PMID: 26341780 Free PMC article. Review.
-
Prostate organogenesis: tissue induction, hormonal regulation and cell type specification.Development. 2017 Apr 15;144(8):1382-1398. doi: 10.1242/dev.148270. Development. 2017. PMID: 28400434 Free PMC article. Review.
-
Noncanonical Wnt/Ror2 Signaling Regulates Basal Cell Fidelity and Branching Morphogenesis in the Mammary Gland.bioRxiv [Preprint]. 2025 Feb 25:2025.02.25.640099. doi: 10.1101/2025.02.25.640099. bioRxiv. 2025. PMID: 40060578 Free PMC article. Preprint.
-
Epigenetic regulation of p63 blocks squamous-to-neuroendocrine transdifferentiation in esophageal development and malignancy.Sci Adv. 2024 Oct 11;10(41):eadq0479. doi: 10.1126/sciadv.adq0479. Epub 2024 Oct 9. Sci Adv. 2024. PMID: 39383220 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

