Matrix gla protein binds to fibronectin and enhances cell attachment and spreading on fibronectin
- PMID: 25210519
- PMCID: PMC4158265
- DOI: 10.1155/2014/807013
Matrix gla protein binds to fibronectin and enhances cell attachment and spreading on fibronectin
Abstract
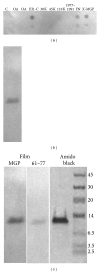
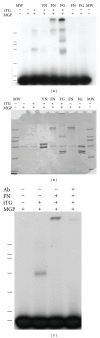
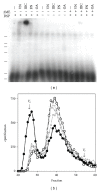
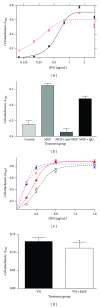
Background. Matrix Gla protein (MGP) is a vitamin K-dependent, extracellular matrix protein. MGP is a calcification inhibitor of arteries and cartilage. However MGP is synthesized in many tissues and is especially enriched in embryonic tissues and in cancer cells. The presence of MGP in those instances does not correlate well with the calcification inhibitory role. This study explores a potential mechanism for MGP to bind to matrix proteins and alter cell matrix interactions. Methods. To determine whether MGP influences cell behavior through interaction with fibronectin, we studied MGP binding to fibronectin, the effect of MGP on fibronectin mediated cell attachment and spreading and immunolocalized MGP and fibronectin. Results. First, MGP binds to fibronectin. The binding site for MGP is in a specific fibronectin fragment, called III1-C or anastellin. The binding site for fibronectin is in a MGP C-terminal peptide comprising amino acids 61-77. Second, MGP enhances cell attachment and cell spreading on fibronectin. MGP alone does not promote cell adhesion. Third, MGP is present in fibronectin-rich regions of tissue sections. Conclusions. MGP binds to fibronectin. The presence of MGP increased cell-fibronectin interactions.
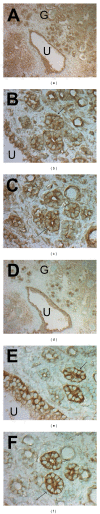
Figures

Similar articles
-
Matrix Gla protein C-terminal region binds to vitronectin. Co-localization suggests binding occurs during tissue development.Matrix Biol. 2005 Aug;24(5):353-61. doi: 10.1016/j.matbio.2005.05.004. Matrix Biol. 2005. PMID: 15982861
-
Purification of matrix Gla protein from a marine teleost fish, Argyrosomus regius: calcified cartilage and not bone as the primary site of MGP accumulation in fish.J Bone Miner Res. 2003 Feb;18(2):244-59. doi: 10.1359/jbmr.2003.18.2.244. J Bone Miner Res. 2003. PMID: 12568402
-
Developmental expression and hormonal regulation of the rat matrix Gla protein (MGP) gene in chondrogenesis and osteogenesis.J Cell Biochem. 1991 Aug;46(4):351-65. doi: 10.1002/jcb.240460410. J Cell Biochem. 1991. PMID: 1757478
-
[The matrix-gla protein awakening may lead to the demise of vascular calcification].Nephrol Ther. 2015 Jul;11(4):191-200. doi: 10.1016/j.nephro.2014.12.003. Epub 2015 Mar 17. Nephrol Ther. 2015. PMID: 25794931 Review. French.
-
The role of Gla proteins in vascular calcification.Crit Rev Eukaryot Gene Expr. 1998;8(3-4):357-75. doi: 10.1615/critreveukargeneexpr.v8.i3-4.60. Crit Rev Eukaryot Gene Expr. 1998. PMID: 9807700 Review.
Cited by
-
Single-cell RNA sequencing of neurofibromas reveals a tumor microenvironment favorable for neural regeneration and immune suppression in a neurofibromatosis type 1 porcine model.Front Oncol. 2023 Sep 25;13:1253659. doi: 10.3389/fonc.2023.1253659. eCollection 2023. Front Oncol. 2023. PMID: 37817770 Free PMC article.
-
Inactive matrix gla protein plasma levels are associated with peripheral neuropathy in Type 2 diabetes.PLoS One. 2020 Feb 24;15(2):e0229145. doi: 10.1371/journal.pone.0229145. eCollection 2020. PLoS One. 2020. PMID: 32092076 Free PMC article.
-
Detection and screening of small molecule agents for overcoming Sorafenib resistance of hepatocellular carcinoma: a bioinformatics study.Int J Clin Exp Med. 2015 Feb 15;8(2):2317-25. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 25932168 Free PMC article.
-
The Role of Matrix Gla Protein (MGP) Expression in Paclitaxel and Topotecan Resistant Ovarian Cancer Cell Lines.Int J Mol Sci. 2018 Sep 25;19(10):2901. doi: 10.3390/ijms19102901. Int J Mol Sci. 2018. PMID: 30257426 Free PMC article.
-
Regulating the cell shift of endothelial cell-like myofibroblasts in pulmonary fibrosis.Eur Respir J. 2023 Jun 8;61(6):2201799. doi: 10.1183/13993003.01799-2022. Print 2023 Jun. Eur Respir J. 2023. PMID: 36758986 Free PMC article.
References
-
- Fraser JD, Price PA. Lung, heart, and kidney express high levels of mRNA for the vitamin K-dependent matrix Gla protein. Implications for the possible functions of matrix Gla protein and for the tissue distribution of the γ-carboxylase. The Journal of Biological Chemistry. 1988;263(23):11033–11036. - PubMed
-
- Zhao J, Araki N, Nishimoto SK. Quantitation of matrix Gla protein mRNA by competitive polymerase chain reaction using glyceraldehyde-3-phosphate dehydrogenase as an internal control. Gene. 1995;155(2):159–165. - PubMed
-
- Zhao J, Nishimoto SK. Matrix Gla protein gene expression is elevated during postnatal development. Matrix Biology. 1996;15(2):131–140. - PubMed
-
- Luo G, Ducy P, McKee MD, et al. Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protien. Nature. 1997;386(6620):78–81. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

