Structure and function of nucleotide sugar transporters: Current progress
- PMID: 25210595
- PMCID: PMC4151994
- DOI: 10.1016/j.csbj.2014.05.003
Structure and function of nucleotide sugar transporters: Current progress
Abstract
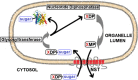
The proteomes of eukaryotes, bacteria and archaea are highly diverse due, in part, to the complex post-translational modification of protein glycosylation. The diversity of glycosylation in eukaryotes is reliant on nucleotide sugar transporters to translocate specific nucleotide sugars that are synthesised in the cytosol and nucleus, into the endoplasmic reticulum and Golgi apparatus where glycosylation reactions occur. Thirty years of research utilising multidisciplinary approaches has contributed to our current understanding of NST function and structure. In this review, the structure and function, with reference to various disease states, of several NSTs including the UDP-galactose, UDP-N-acetylglucosamine, UDP-N-acetylgalactosamine, GDP-fucose, UDP-N-acetylglucosamine/UDP-glucose/GDP-mannose and CMP-sialic acid transporters will be described. Little is known regarding the exact structure of NSTs due to difficulties associated with crystallising membrane proteins. To date, no three-dimensional structure of any NST has been elucidated. What is known is based on computer predictions, mutagenesis experiments, epitope-tagging studies, in-vitro assays and phylogenetic analysis. In this regard the best-characterised NST to date is the CMP-sialic acid transporter (CST). Therefore in this review we will provide the current state-of-play with respect to the structure-function relationship of the (CST). In particular we have summarised work performed by a number groups detailing the affect of various mutations on CST transport activity, efficiency, and substrate specificity.
Keywords: CMP-sialic acid transporter; Endoplasmic reticulum; Golgi apparatus; Nucleotide sugar transporters; STD NMR spectroscopy.
Figures


References
-
- Hayashi H., Yamashita Y. Role of N-glycosylation in cell surface expression and protection against proteolysis of the intestinal anion exchanger SLC26A3. Am J Physiol Cell Physiol. 2012;302:C781–C795. - PubMed
-
- van Kooyk Y., Rabinovich G.A. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat Immunol. 2008;9:593–601. - PubMed
-
- Zhou F., Xu W., Hong M., Pan Z., Sinko P.J., Ma J. The role of N-linked glycosylation in protein folding, membrane targeting, and substrate binding of human organic anion transporter hOAT4. Mol Pharmacol. 2005;67:868–876. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
