The nuclear exosome is active and important during budding yeast meiosis
- PMID: 25210768
- PMCID: PMC4161446
- DOI: 10.1371/journal.pone.0107648
The nuclear exosome is active and important during budding yeast meiosis
Abstract
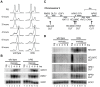
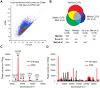
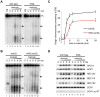
Nuclear RNA degradation pathways are highly conserved across eukaryotes and play important roles in RNA quality control. Key substrates for exosomal degradation include aberrant functional RNAs and cryptic unstable transcripts (CUTs). It has recently been reported that the nuclear exosome is inactivated during meiosis in budding yeast through degradation of the subunit Rrp6, leading to the stabilisation of a subset of meiotic unannotated transcripts (MUTs) of unknown function. We have analysed the activity of the nuclear exosome during meiosis by deletion of TRF4, which encodes a key component of the exosome targeting complex TRAMP. We find that TRAMP mutants produce high levels of CUTs during meiosis that are undetectable in wild-type cells, showing that the nuclear exosome remains functional for CUT degradation, and we further report that the meiotic exosome complex contains Rrp6. Indeed Rrp6 over-expression is insufficient to suppress MUT transcripts, showing that the reduced amount of Rrp6 in meiotic cells does not directly cause MUT accumulation. Lack of TRAMP activity stabilises ∼ 1600 CUTs in meiotic cells, which occupy 40% of the binding capacity of the nuclear cap binding complex (CBC). CBC mutants display defects in the formation of meiotic double strand breaks (DSBs), and we see similar defects in TRAMP mutants, suggesting that a key function of the nuclear exosome is to prevent saturation of the CBC complex by CUTs. Together, our results show that the nuclear exosome remains active in meiosis and has an important role in facilitating meiotic recombination.
Conflict of interest statement
Figures

Similar articles
-
TRAMP complex enhances RNA degradation by the nuclear exosome component Rrp6.J Biol Chem. 2010 Feb 5;285(6):3540-3547. doi: 10.1074/jbc.M109.058396. Epub 2009 Dec 2. J Biol Chem. 2010. PMID: 19955569 Free PMC article.
-
Nab3 facilitates the function of the TRAMP complex in RNA processing via recruitment of Rrp6 independent of Nrd1.PLoS Genet. 2015 Mar 16;11(3):e1005044. doi: 10.1371/journal.pgen.1005044. eCollection 2015 Mar. PLoS Genet. 2015. PMID: 25775092 Free PMC article.
-
The fission yeast MTREC complex targets CUTs and unspliced pre-mRNAs to the nuclear exosome.Nat Commun. 2015 May 20;6:7050. doi: 10.1038/ncomms8050. Nat Commun. 2015. PMID: 25989903 Free PMC article.
-
Nuclear RNA surveillance: role of TRAMP in controlling exosome specificity.Wiley Interdiscip Rev RNA. 2013 Mar-Apr;4(2):217-31. doi: 10.1002/wrna.1155. Epub 2013 Feb 15. Wiley Interdiscip Rev RNA. 2013. PMID: 23417976 Free PMC article. Review.
-
Comparison of the yeast and human nuclear exosome complexes.Biochem Soc Trans. 2012 Aug;40(4):850-5. doi: 10.1042/BST20120061. Biochem Soc Trans. 2012. PMID: 22817747 Review.
Cited by
-
Widespread exon skipping triggers degradation by nuclear RNA surveillance in fission yeast.Genome Res. 2015 Jun;25(6):884-96. doi: 10.1101/gr.185371.114. Epub 2015 Apr 16. Genome Res. 2015. PMID: 25883323 Free PMC article.
-
Tri-methylation of histone H3 lysine 4 facilitates gene expression in ageing cells.Elife. 2018 Oct 2;7:e34081. doi: 10.7554/eLife.34081. Elife. 2018. PMID: 30274593 Free PMC article.
-
Surveillance-ready transcription: nuclear RNA decay as a default fate.Open Biol. 2018 Mar;8(3):170270. doi: 10.1098/rsob.170270. Open Biol. 2018. PMID: 29563193 Free PMC article. Review.
-
Rrp6: Integrated roles in nuclear RNA metabolism and transcription termination.Wiley Interdiscip Rev RNA. 2016 Jan-Feb;7(1):91-104. doi: 10.1002/wrna.1317. Epub 2015 Nov 26. Wiley Interdiscip Rev RNA. 2016. PMID: 26612606 Free PMC article. Review.
-
Long noncoding RNA repertoire and targeting by nuclear exosome, cytoplasmic exonuclease, and RNAi in fission yeast.RNA. 2018 Sep;24(9):1195-1213. doi: 10.1261/rna.065524.118. Epub 2018 Jun 18. RNA. 2018. PMID: 29914874 Free PMC article.
References
-
- Mitchell P, Petfalski E, Shevchenko A, Mann M, Tollervey D (1997) The exosome: a conserved eukaryotic RNA processing complex containing multiple 3'—>5' exoribonucleases. Cell 91: 457–466. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

