Cell therapy with embryonic stem cell-derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair
- PMID: 25210842
- PMCID: PMC4212793
- DOI: 10.1021/nn504617g
Cell therapy with embryonic stem cell-derived cardiomyocytes encapsulated in injectable nanomatrix gel enhances cell engraftment and promotes cardiac repair
Abstract
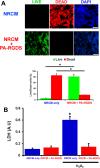
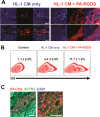
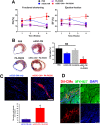
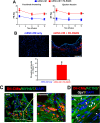
A significant barrier to the therapeutic use of stem cells is poor cell retention in vivo. Here, we evaluate the therapeutic potential and long-term engraftment of cardiomyocytes (CMs) derived from mouse embryonic stem cells (mESCs) encapsulated in an injectable nanomatrix gel consisting of peptide amphiphiles incorporating cell adhesive ligand Arg-Gly-Asp-Ser (PA-RGDS) in experimental myocardial infarction (MI). We cultured rat neonatal CMs in PA-RGDS for 7 days and found that more than 90% of the CMs survived. Next, we intramyocardially injected mouse CM cell line HL-1 CMs with or without PA-RGDS into uninjured hearts. Histologic examination and flow cytometry analysis of digested heart tissues showed approximately 3-fold higher engraftment in the mice that received CMs with PA-RGDS compared to those without PA-RGDS. We further investigated the therapeutic effects and long-term engraftment of mESC-CMs with PA-RGDS on MI in comparison with PBS control, CM-only, and PA-RGDS only. Echocardiography demonstrated that the CM-only and CM+PA-RGDS groups showed higher cardiac function at week 2 compared to other groups. However, from 3 weeks, higher cardiac function was maintained only in the CM+PA-RGDS group; this was sustained for 12 weeks. Confocal microscopic examination of the cardiac tissues harvested at 14 weeks demonstrated sustained engraftment and integration of mESC-CMs into host myocardium in the CM+PA-RGDS group only. This study for the first time demonstrated that PA-RGDS encapsulation can enhance survival of mESC-derived CMs and improve cardiac function post-MI. This nanomatrix gel-mediated stem cell therapy can be a promising option for treating MI.
Keywords: PA-RGDS; cardiac regeneration; cardiomyocyte; myocardial infarction; pluripotent stem cell.
Figures


Similar articles
-
Enhanced Therapeutic and Long-Term Dynamic Vascularization Effects of Human Pluripotent Stem Cell-Derived Endothelial Cells Encapsulated in a Nanomatrix Gel.Circulation. 2017 Nov 14;136(20):1939-1954. doi: 10.1161/CIRCULATIONAHA.116.026329. Epub 2017 Sep 29. Circulation. 2017. PMID: 28972000 Free PMC article.
-
Thymosin β4 increases cardiac cell proliferation, cell engraftment, and the reparative potency of human induced-pluripotent stem cell-derived cardiomyocytes in a porcine model of acute myocardial infarction.Theranostics. 2021 Jun 26;11(16):7879-7895. doi: 10.7150/thno.56757. eCollection 2021. Theranostics. 2021. PMID: 34335970 Free PMC article.
-
Dual stem cell therapy synergistically improves cardiac function and vascular regeneration following myocardial infarction.Nat Commun. 2019 Jul 16;10(1):3123. doi: 10.1038/s41467-019-11091-2. Nat Commun. 2019. PMID: 31311935 Free PMC article.
-
Pluripotent stem cells for cardiac regeneration: overview of recent advances & emerging trends.Indian J Med Res. 2013 Feb;137(2):270-82. Indian J Med Res. 2013. PMID: 23563370 Free PMC article. Review.
-
Evaluation of Multiple Biological Therapies for Ischemic Cardiac Disease.Cell Transplant. 2016;25(9):1591-1607. doi: 10.3727/096368916X691501. Epub 2016 May 10. Cell Transplant. 2016. PMID: 27165370 Review.
Cited by
-
Generation of Human Pluripotent Stem Cell-derived Endothelial Cells and Their Therapeutic Utility.Curr Cardiol Rep. 2018 May 5;20(6):45. doi: 10.1007/s11886-018-0985-8. Curr Cardiol Rep. 2018. PMID: 29730842 Free PMC article. Review.
-
Innovations in Disease State Responsive Soft Materials for Targeting Extracellular Stimuli Associated with Cancer, Cardiovascular Disease, Diabetes, and Beyond.Adv Mater. 2021 Nov;33(46):e2007504. doi: 10.1002/adma.202007504. Epub 2021 Jun 17. Adv Mater. 2021. PMID: 34145625 Free PMC article. Review.
-
Recent Advances in Metal-Containing Polymer Hydrogels.Macromol Rapid Commun. 2017 Jul;38(14):10.1002/marc.201700109. doi: 10.1002/marc.201700109. Epub 2017 May 26. Macromol Rapid Commun. 2017. PMID: 28547817 Free PMC article. Review.
-
Regenerative loss in the animal kingdom as viewed from the mouse digit tip and heart.Dev Biol. 2024 Mar;507:44-63. doi: 10.1016/j.ydbio.2023.12.008. Epub 2023 Dec 24. Dev Biol. 2024. PMID: 38145727 Free PMC article. Review.
-
Position Paper of the European Society of Cardiology Working Group Cellular Biology of the Heart: cell-based therapies for myocardial repair and regeneration in ischemic heart disease and heart failure.Eur Heart J. 2016 Jun 14;37(23):1789-98. doi: 10.1093/eurheartj/ehw113. Epub 2016 Apr 7. Eur Heart J. 2016. PMID: 27055812 Free PMC article. Review.
References
-
- Takahashi K.; Tanabe K.; Ohnuki M.; Narita M.; Ichisaka T.; Tomoda K.; Yamanaka S. Induction of Pluripotent Stem Cells from Adult Human Fibroblasts by Defined Factors. Cell 2007, 131, 861–872. - PubMed
-
- Zwi L.; Caspi O.; Arbel G.; Huber I.; Gepstein A.; Park I. H.; Gepstein L. Cardiomyocyte Differentiation of Human Induced Pluripotent Stem Cells. Circulation 2009, 120, 1513–1523. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

