Mathematical modeling of bacterial kinetics to predict the impact of antibiotic colonic exposure and treatment duration on the amount of resistant enterobacteria excreted
- PMID: 25210849
- PMCID: PMC4161292
- DOI: 10.1371/journal.pcbi.1003840
Mathematical modeling of bacterial kinetics to predict the impact of antibiotic colonic exposure and treatment duration on the amount of resistant enterobacteria excreted
Abstract
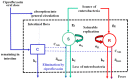
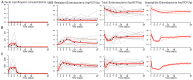
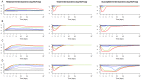
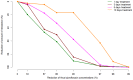
Fecal excretion of antibiotics and resistant bacteria in the environment are major public health threats associated with extensive farming and modern medical care. Innovative strategies that can reduce the intestinal antibiotic concentrations during treatments are in development. However, the effect of lower exposure on the amount of resistant enterobacteria excreted has not been quantified, making it difficult to anticipate the impact of these strategies. Here, we introduce a bacterial kinetic model to capture the complex relationships between drug exposure, loss of susceptible enterobacteria and growth of resistant strains in the feces of piglets receiving placebo, 1.5 or 15 mg/kg/day ciprofloxacin, a fluoroquinolone, for 5 days. The model could well describe the kinetics of drug susceptible and resistant enterobacteria observed during treatment, and up to 22 days after treatment cessation. Next, the model was used to predict the expected amount of resistant enterobacteria excreted over an average piglet's lifetime (150 days) when varying drug exposure and treatment duration. For the clinically relevant dose of 15 mg/kg/day for 5 days, the total amount of resistant enterobacteria excreted was predicted to be reduced by 75% and 98% when reducing treatment duration to 3 and 1 day treatment, respectively. Alternatively, for a fixed 5-days treatment, the level of resistance excreted could be reduced by 18%, 33%, 57.5% and 97% if 3, 5, 10 and 30 times lower levels of colonic drug concentrations were achieved, respectively. This characterization on in vivo data of the dynamics of resistance to antibiotics in the colonic flora could provide new insights into the mechanism of dissemination of resistance and can be used to design strategies aiming to reduce it.
Conflict of interest statement
We have read the journal's policy and have the following conflicts: Thu Thuy Nguyen performed statistical work for the Da Volterra Company through a contract with UMR 738 INSERM and University Paris Diderot. Elisabeth Chachaty is a consultant for the Da Volterra Company. Antoine Andremont is a scientific adviser of the Da Volterra Company within the framework of the French law on Innovation and Research. France Mentré is a consultant for the Da Volterra Company.
Figures
Similar articles
-
Correlation between fecal concentrations of ciprofloxacin and fecal counts of resistant Enterobacteriaceae in piglets treated with ciprofloxacin: toward new means to control the spread of resistance?Antimicrob Agents Chemother. 2012 Sep;56(9):4973-5. doi: 10.1128/AAC.06402-11. Epub 2012 Jul 2. Antimicrob Agents Chemother. 2012. PMID: 22751547 Free PMC article.
-
Changes in concentrations of fluoroquinolones and of ciprofloxacin-resistant Enterobacteriaceae in chicken feces and manure stored in a heap.J Environ Qual. 2012 May-Jun;41(3):754-63. doi: 10.2134/jeq2011.0313. J Environ Qual. 2012. PMID: 22565257 Clinical Trial.
-
Ciprofloxacin-resistant enterobacteria harboring the aac(6')-Ib-cr variant isolated from feces of inpatients in an intensive care unit in Uruguay.Antimicrob Agents Chemother. 2008 Feb;52(2):806-7. doi: 10.1128/AAC.00444-07. Epub 2007 Nov 12. Antimicrob Agents Chemother. 2008. PMID: 17999962 Free PMC article. No abstract available.
-
Impact of extensive antibiotic treatment on faecal carriage of antibiotic-resistant enterobacteria in children in a low resistance prevalence setting.PLoS One. 2017 Nov 7;12(11):e0187618. doi: 10.1371/journal.pone.0187618. eCollection 2017. PLoS One. 2017. PMID: 29112974 Free PMC article.
-
[Sensitivity of opportunistic-pathogenic microflora isolated from humans to antibiotics before and after a stay in a hermetically closed chamber].Kosm Biol Aviakosm Med. 1989 May-Jun;23(3):62-5. Kosm Biol Aviakosm Med. 1989. PMID: 2569546 Russian.
Cited by
-
Impact of Antibiotic Gut Exposure on the Temporal Changes in Microbiome Diversity.Antimicrob Agents Chemother. 2019 Sep 23;63(10):e00820-19. doi: 10.1128/AAC.00820-19. Print 2019 Oct. Antimicrob Agents Chemother. 2019. PMID: 31307985 Free PMC article. Clinical Trial.
-
Quantifying trade-offs between therapeutic efficacy and resistance dissemination for enrofloxacin dose regimens in cattle.Sci Rep. 2024 Sep 4;14(1):20598. doi: 10.1038/s41598-024-70741-8. Sci Rep. 2024. PMID: 39232037 Free PMC article.
-
A stochastic analysis of the interplay between antibiotic dose, mode of action, and bacterial competition in the evolution of antibiotic resistance.PLoS Comput Biol. 2023 Aug 14;19(8):e1011364. doi: 10.1371/journal.pcbi.1011364. eCollection 2023 Aug. PLoS Comput Biol. 2023. PMID: 37578976 Free PMC article.
-
Low-Concentration Ciprofloxacin Selects Plasmid-Mediated Quinolone Resistance Encoding Genes and Affects Bacterial Taxa in Soil Containing Manure.Front Microbiol. 2016 Nov 1;7:1730. doi: 10.3389/fmicb.2016.01730. eCollection 2016. Front Microbiol. 2016. PMID: 27847506 Free PMC article.
-
Send more data: a systematic review of mathematical models of antimicrobial resistance.Antimicrob Resist Infect Control. 2018 Sep 29;7:117. doi: 10.1186/s13756-018-0406-1. eCollection 2018. Antimicrob Resist Infect Control. 2018. PMID: 30288257 Free PMC article.
References
-
- Zhou L-J, Ying G-G, Liu S, Zhang R-Q, Lai H-J, Chen Z-F, Pan C-G (2013) Excretion masses and environmental occurrence of antibiotics in typical swine and dairy cattle farms in China. Sci Total Environ 444: 183–195. - PubMed
-
- Li Y-X, Zhang X-L, Li W, Lu X-F, Liu B, Wang J (2013) The residues and environmental risks of multiple veterinary antibiotics in animal faeces. Environ Monit Assess 85 (3) 2211–2220. - PubMed
-
- Aarestrup FM, Seyfarth AM, Emborg H-D, Pedersen K, Hendriksen RS, Bager F (2001) Effect of Abolishment of the Use of Antimicrobial Agents for Growth Promotion on Occurrence of Antimicrobial Resistance in Fecal Enterococci from Food Animals in Denmark. Antimicrob Agents Chemother 45 (7) 2054–2059. - PMC - PubMed
-
- Risk Response Network (2013) The Dangers of Hubris on Human Health. Global Risk Report Eighth Edition, World Economic Forum.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

