Cleavage specificity analysis of six type II transmembrane serine proteases (TTSPs) using PICS with proteome-derived peptide libraries
- PMID: 25211023
- PMCID: PMC4161349
- DOI: 10.1371/journal.pone.0105984
Cleavage specificity analysis of six type II transmembrane serine proteases (TTSPs) using PICS with proteome-derived peptide libraries
Abstract
Background: Type II transmembrane serine proteases (TTSPs) are a family of cell membrane tethered serine proteases with unclear roles as their cleavage site specificities and substrate degradomes have not been fully elucidated. Indeed just 52 cleavage sites are annotated in MEROPS, the database of proteases, their substrates and inhibitors.
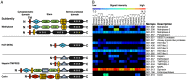
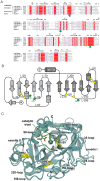
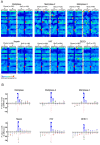
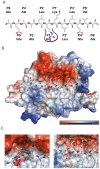
Methodology/principal finding: To profile the active site specificities of the TTSPs, we applied Proteomic Identification of protease Cleavage Sites (PICS). Human proteome-derived database searchable peptide libraries were assayed with six human TTSPs (matriptase, matriptase-2, matriptase-3, HAT, DESC and hepsin) to simultaneously determine sequence preferences on the N-terminal non-prime (P) and C-terminal prime (P') sides of the scissile bond. Prime-side cleavage products were isolated following biotinylation and identified by tandem mass spectrometry. The corresponding non-prime side sequences were derived from human proteome databases using bioinformatics. Sequencing of 2,405 individual cleaved peptides allowed for the development of the family consensus protease cleavage site specificity revealing a strong specificity for arginine in the P1 position and surprisingly a lysine in P1' position. TTSP cleavage between R↓K was confirmed using synthetic peptides. By parsing through known substrates and known structures of TTSP catalytic domains, and by modeling the remainder, structural explanations for this strong specificity were derived.
Conclusions: Degradomics analysis of 2,405 cleavage sites revealed a similar and characteristic TTSP family specificity at the P1 and P1' positions for arginine and lysine in unfolded peptides. The prime side is important for cleavage specificity, thus making these proteases unusual within the tryptic-enzyme class that generally has overriding non-prime side specificity.
Conflict of interest statement
Figures

References
-
- Leytus SP, Loeb KR, Hagen FS, Kurachi K, Davie EW (1988) A novel trypsin-like serine protease (hepsin) with a putative transmembrane domain expressed by human liver and hepatoma cells. Biochemistry 27: 1067–1074. - PubMed
-
- Netzel-Arnett S, Hooper JD, Szabo R, Madison EL, Quigley JP, et al. (2003) Membrane anchored serine proteases: a rapidly expanding group of cell surface proteolytic enzymes with potential roles in cancer. Cancer Metastasis Rev 22: 237–258. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

