Sialylation of prion protein controls the rate of prion amplification, the cross-species barrier, the ratio of PrPSc glycoform and prion infectivity
- PMID: 25211026
- PMCID: PMC4161476
- DOI: 10.1371/journal.ppat.1004366
Sialylation of prion protein controls the rate of prion amplification, the cross-species barrier, the ratio of PrPSc glycoform and prion infectivity
Abstract
The central event underlying prion diseases involves conformational change of the cellular form of the prion protein (PrP(C)) into the disease-associated, transmissible form (PrP(Sc)). Pr(PC) is a sialoglycoprotein that contains two conserved N-glycosylation sites. Among the key parameters that control prion replication identified over the years are amino acid sequence of host PrP(C) and the strain-specific structure of PrPSc. The current work highlights the previously unappreciated role of sialylation of PrP(C) glycans in prion pathogenesis, including its role in controlling prion replication rate, infectivity, cross-species barrier and PrP(Sc) glycoform ratio. The current study demonstrates that undersialylated PrP(C) is selected during prion amplification in Protein Misfolding Cyclic Amplification (PMCAb) at the expense of oversialylated PrP(C). As a result, PMCAb-derived PrP(Sc) was less sialylated than brain-derived PrP(Sc). A decrease in PrPSc sialylation correlated with a drop in infectivity of PMCAb-derived material. Nevertheless, enzymatic de-sialylation of PrP(C) using sialidase was found to increase the rate of PrP(Sc) amplification in PMCAb from 10- to 10,000-fold in a strain-dependent manner. Moreover, de-sialylation of PrP(C) reduced or eliminated a species barrier of for prion amplification in PMCAb. These results suggest that the negative charge of sialic acid controls the energy barrier of homologous and heterologous prion replication. Surprisingly, the sialylation status of PrP(C) was also found to control PrP(Sc) glycoform ratio. A decrease in Pr(PC) sialylation levels resulted in a higher percentage of the diglycosylated glycoform in PrP(Sc). 2D analysis of charge distribution revealed that the sialylation status of brain-derived PrP(C) differed from that of spleen-derived PrP(C). Knocking out lysosomal sialidase Neu1 did not change the sialylation status of brain-derived PrP(C), suggesting that Neu1 is not responsible for desialylation of PrP(C). The current work highlights previously unappreciated role of PrP(C) sialylation in prion diseases and opens multiple new research directions, including development of new therapeutic approaches.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



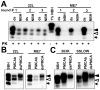


References
-
- Cohen FE, Prusiner SB (1998) Pathologic conformations of prion proteins. Annu Rev Biochem 67: 793–819. - PubMed
-
- Stahl N, Baldwin MA, Hecker R, Pan KM, Burlingame AL, et al. (1992) Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry 31: 5043–5053. - PubMed
-
- Stahl N, Borchelt DR, Hsiao K, Prusiner SB (1987) Scrapie prion protein contains a phosphatidylinositol glycolipid. Cell 51: 229–240. - PubMed
-
- Turk E, Teplow DB, Hood LE, Prusiner SB (1988) Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem 176: 21–30. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

