Retroviral retention activates a Syk-dependent HemITAM in human tetherin
- PMID: 25211072
- PMCID: PMC4161388
- DOI: 10.1016/j.chom.2014.08.005
Retroviral retention activates a Syk-dependent HemITAM in human tetherin
Abstract
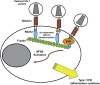
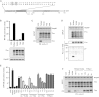
Tetherin (BST2/CD317) restricts the release of enveloped viral particles from infected cells. Coupled to this virion retention, hominid tetherins induce proinflammatory gene expression via activating NF-κB. We investigated the events initiating this tetherin-induced signaling and show that physical retention of retroviral particles induces the phosphorylation of conserved tyrosine residues in the cytoplasmic tails of tetherin dimers. This phosphorylation induces the recruitment of spleen tyrosine kinase (Syk), which is required for downstream NF-κB activation, indicating that the tetherin cytoplasmic tail resembles the hemi-immunoreceptor tyrosine-based activation motifs (hemITAMs) found in C-type lectin pattern recognition receptors. Retroviral-induced tetherin signaling is coupled to the cortical actin cytoskeleton via the Rac-GAP-containing protein RICH2 (ARHGAP44), and a naturally occurring tetherin polymorphism with reduced RICH2 binding exhibits decreased phosphorylation and NF-κB activation. Thus, upon virion retention, this linkage to the actin cytoskeleton likely triggers tetherin phosphorylation and subsequent signal transduction to induce an antiviral state.
Copyright © 2014 The Authors. Published by Elsevier Inc. All rights reserved.
Figures






Comment in
-
Tethering viral restriction to signal transduction.Cell Host Microbe. 2014 Sep 10;16(3):267-9. doi: 10.1016/j.chom.2014.08.013. Cell Host Microbe. 2014. PMID: 25211067
References
-
- Chu D.H., Morita C.T., Weiss A. The Syk family of protein tyrosine kinases in T-cell activation and development. Immunol. Rev. 1998;165:167–180. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

