Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells
- PMID: 25211170
- PMCID: PMC4206665
- DOI: 10.1097/ALN.0000000000000446
Cell-based therapy for acute organ injury: preclinical evidence and ongoing clinical trials using mesenchymal stem cells
Abstract
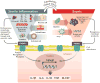
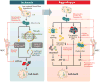
Critically ill patients often suffer from multiple organ failures involving lung, kidney, liver, or brain. Genomic, proteomic, and metabolomic approaches highlight common injury mechanisms leading to acute organ failure. This underlines the need to focus on therapeutic strategies affecting multiple injury pathways. The use of adult stem cells such as mesenchymal stem or stromal cells (MSC) may represent a promising new therapeutic approach as increasing evidence shows that MSC can exert protective effects following injury through the release of promitotic, antiapoptotic, antiinflammatory, and immunomodulatory soluble factors. Furthermore, they can mitigate metabolomic and oxidative stress imbalance. In this work, the authors review the biological capabilities of MSC and the results of clinical trials using MSC as therapy in acute organ injuries. Although preliminary results are encouraging, more studies concerning safety and efficacy of MSC therapy are needed to determine their optimal clinical use. (ANESTHESIOLOGY 2014; 121:1099-121).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, Schein RM, Sibbald WJ. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101:1644–55. - PubMed
-
- Awad SS. State-of-the-art therapy for severe sepsis and multisystem organ dysfunction. Am J Surg. 2003;186:S23–30. - PubMed
-
- Vincent J-L, Sakr Y, Sprung CL, Ranieri VM, Reinhart K, Gerlach H, Moreno R, Carlet J, Le Gall J-R, Payen D Investigators SOiAIP. Sepsis in European intensive care units: Results of the SOAP study. Crit Care Med. 2006;34:344–53. - PubMed
-
- Uchino S, Kellum MJA, Bellomo MR, Doig MGS, Morimatsu PH, Morgera MS, Schetz MM, Tan M, Bouman M, Macedo ME, Gibney MN, Tolwani MA, Ronco MC. Acute Renal Failure in Critically Ill Patients: A Multinational, Multicenter Study. JAMA. 2005;294:813–8. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

