Plasmodium falciparum infection induces expression of a mosquito salivary protein (Agaphelin) that targets neutrophil function and inhibits thrombosis without impairing hemostasis
- PMID: 25211214
- PMCID: PMC4161438
- DOI: 10.1371/journal.ppat.1004338
Plasmodium falciparum infection induces expression of a mosquito salivary protein (Agaphelin) that targets neutrophil function and inhibits thrombosis without impairing hemostasis
Abstract
Background: Invasion of mosquito salivary glands (SGs) by Plasmodium falciparum sporozoites is an essential step in the malaria life cycle. How infection modulates gene expression, and affects hematophagy remains unclear.
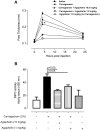
Principal findings: Using Affimetrix chip microarray, we found that at least 43 genes are differentially expressed in the glands of Plasmodium falciparum-infected Anopheles gambiae mosquitoes. Among the upregulated genes, one codes for Agaphelin, a 58-amino acid protein containing a single Kazal domain with a Leu in the P1 position. Agaphelin displays high homology to orthologs present in Aedes sp and Culex sp salivary glands, indicating an evolutionarily expanded family. Kinetics and surface plasmon resonance experiments determined that chemically synthesized Agaphelin behaves as a slow and tight inhibitor of neutrophil elastase (K(D) ∼ 10 nM), but does not affect other enzymes, nor promotes vasodilation, or exhibit antimicrobial activity. TAXIscan chamber assay revealed that Agaphelin inhibits neutrophil chemotaxis toward fMLP, affecting several parameter associated with cell migration. In addition, Agaphelin reduces paw edema formation and accumulation of tissue myeloperoxidase triggered by injection of carrageenan in mice. Agaphelin also blocks elastase/cathepsin-mediated platelet aggregation, abrogates elastase-mediated cleavage of tissue factor pathway inhibitor, and attenuates neutrophil-induced coagulation. Notably, Agaphelin inhibits neutrophil extracellular traps (NETs) formation and prevents FeCl3-induced arterial thrombosis, without impairing hemostasis.
Conclusions: Blockade of neutrophil elastase emerges as a novel antihemostatic mechanism in hematophagy; it also supports the notion that neutrophils and the innate immune response are targets for antithrombotic therapy. In addition, Agaphelin is the first antihemostatic whose expression is induced by Plasmodium sp infection. These results suggest that an important interplay takes place in parasite-vector-host interactions.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures






Similar articles
-
Salivary gland transcriptome analysis during Plasmodium infection in malaria vector Anopheles stephensi.Int J Infect Dis. 2009 Sep;13(5):636-46. doi: 10.1016/j.ijid.2008.07.027. Epub 2009 Jan 6. Int J Infect Dis. 2009. PMID: 19128996
-
Identification and molecular characterization of a novel protein Saglin as a target of monoclonal antibodies affecting salivary gland infectivity of Plasmodium sporozoites.Insect Mol Biol. 2007 Dec;16(6):711-22. doi: 10.1111/j.1365-2583.2007.00765.x. Insect Mol Biol. 2007. PMID: 18093000
-
Anopheles gambiae PRS1 modulates Plasmodium development at both midgut and salivary gland steps.PLoS One. 2010 Jul 12;5(7):e11538. doi: 10.1371/journal.pone.0011538. PLoS One. 2010. PMID: 20634948 Free PMC article.
-
Salivary gland-specific gene expression in the malaria vector Anopheles gambiae.Parassitologia. 1999 Sep;41(1-3):483-7. Parassitologia. 1999. PMID: 10697906 Review.
-
The journey of the malaria parasite in the mosquito: hopes for the new century.Parasitol Today. 2000 May;16(5):196-201. doi: 10.1016/s0169-4758(99)01626-9. Parasitol Today. 2000. PMID: 10782078 Review.
Cited by
-
Neutrophils and Malaria.Front Immunol. 2018 Dec 19;9:3005. doi: 10.3389/fimmu.2018.03005. eCollection 2018. Front Immunol. 2018. PMID: 30619354 Free PMC article. Review.
-
Association of neutrophil extracellular traps with the production of circulating DNA in patients with colorectal cancer.iScience. 2022 Jan 30;25(2):103826. doi: 10.1016/j.isci.2022.103826. eCollection 2022 Feb 18. iScience. 2022. PMID: 35198886 Free PMC article.
-
Neutrophil Extracellular Traps and Its Implications in Inflammation: An Overview.Front Immunol. 2017 Feb 6;8:81. doi: 10.3389/fimmu.2017.00081. eCollection 2017. Front Immunol. 2017. PMID: 28220120 Free PMC article. Review.
-
Beyond the bite: how mosquito salivary proteins modulate midgut biology and malaria parasite transmission.Curr Opin Insect Sci. 2025 Jun;69:101363. doi: 10.1016/j.cois.2025.101363. Epub 2025 Mar 11. Curr Opin Insect Sci. 2025. PMID: 40081801 Review.
-
The Role of Thrombo-inflammation in Ischemic Stroke: Focus on the Manipulation and Clinical Application.Mol Neurobiol. 2025 Feb;62(2):2362-2375. doi: 10.1007/s12035-024-04397-w. Epub 2024 Aug 6. Mol Neurobiol. 2025. PMID: 39107669 Review.
References
-
- Ribeiro JM, Francischetti IM (2003) Role of arthropod saliva in blood feeding: sialome and post-sialome perspectives. Annu Rev Entomol 48: 73–88. - PubMed
-
- Schulz C, Engelmann B, Massberg S (2013) Crossroads of coagulation and innate immunity: the case of deep vein thrombosis. J Thromb Haemost 11 Suppl 1: 233–241. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

