Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+T cells targeting AMA1 class I epitopes
- PMID: 25211344
- PMCID: PMC4161338
- DOI: 10.1371/journal.pone.0106241
Sterile immunity to malaria after DNA prime/adenovirus boost immunization is associated with effector memory CD8+T cells targeting AMA1 class I epitopes
Abstract
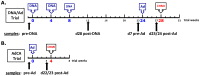
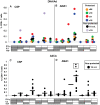
Background: Fifteen volunteers were immunized with three doses of plasmid DNA encoding P. falciparum circumsporozoite protein (CSP) and apical membrane antigen-1 (AMA1) and boosted with human adenovirus-5 (Ad) expressing the same antigens (DNA/Ad). Four volunteers (27%) demonstrated sterile immunity to controlled human malaria infection and, overall, protection was statistically significantly associated with ELISpot and CD8+ T cell IFN-γ activities to AMA1 but not CSP. DNA priming was required for protection, as 18 additional subjects immunized with Ad alone (AdCA) did not develop sterile protection.
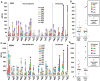
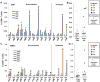
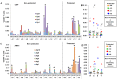
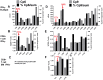
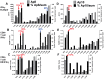
Methodology/principal findings: We sought to identify correlates of protection, recognizing that DNA-priming may induce different responses than AdCA alone. Among protected volunteers, two and three had higher ELISpot and CD8+ T cell IFN-γ responses to CSP and AMA1, respectively, than non-protected volunteers. Unexpectedly, non-protected volunteers in the AdCA trial showed ELISpot and CD8+ T cell IFN-γ responses to AMA1 equal to or higher than the protected volunteers. T cell functionality assessed by intracellular cytokine staining for IFN-γ, TNF-α and IL-2 likewise did not distinguish protected from non-protected volunteers across both trials. However, three of the four protected volunteers showed higher effector to central memory CD8+ T cell ratios to AMA1, and one of these to CSP, than non-protected volunteers for both antigens. These responses were focused on discrete regions of CSP and AMA1. Class I epitopes restricted by A*03 or B*58 supertypes within these regions of AMA1 strongly recalled responses in three of four protected volunteers. We hypothesize that vaccine-induced effector memory CD8+ T cells recognizing a single class I epitope can confer sterile immunity to P. falciparum in humans.
Conclusions/significance: We suggest that better understanding of which epitopes within malaria antigens can confer sterile immunity and design of vaccine approaches that elicit responses to these epitopes will increase the potency of next generation gene-based vaccines.
Trial registration: ClinicalTrials.gov NCT00392015 NCT00870987.
Conflict of interest statement
Figures

References
-
- Murray CJ, Rosenfeld LC, Lim SS, Andrews KG, Foreman KJ, et al. (2012) Global malaria mortality between 1980 and 2010: a systematic analysis. Lancet 379: 413–431. - PubMed
-
- Das P, Horton R (2010) Malaria elimination: worthy, challenging, and just possible. Lancet 376: 1515–1517. - PubMed
-
- Weiss WR, Good MF, Hollingdale MR, Miller LH, Berzofsky JA (1989) Genetic control of immunity to Plasmodium yoelii sporozoites. J Immunol 143: 4263–4266. - PubMed
-
- Hoffman SL, Weiss W, Mellouk S, Sedegah M (1990) Irradiated sporozoite vaccine induces cytotoxic T lymphocytes that recognize malaria antigens on the surface of infected hepatocytes. Immunol Lett 25: 33–38. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

