Antidepressant treatment for postnatal depression
- PMID: 25211400
- PMCID: PMC10589908
- DOI: 10.1002/14651858.CD002018.pub2
Antidepressant treatment for postnatal depression
Abstract
Background: Postnatal depression is a common disorder that can have adverse short- and long-term effects on maternal morbidity, the new infant and the family as a whole. Treatment is often largely by social support and psychological interventions. It is not known whether antidepressants are an effective and safe choice for treatment of this disorder. This review was undertaken to evaluate the effectiveness of different antidepressants and to compare their effectiveness with other forms of treatment, placebo or treatment as usual. It is an update of a review first published in 2001.
Objectives: To assess the effectiveness of antidepressant drugs in comparison with any other treatment (psychological, psychosocial or pharmacological), placebo or treatment as usual for postnatal depression.
Search methods: We searched the Cochrane Depression, Anxiety and Neurosis Group's Specialized Register (CCDANCTR) to 11 July 2014. This register contains reports of relevant randomised controlled trials (RCTs) from the following bibliographic databases: The Cochrane Library (all years), MEDLINE (1950 to date), EMBASE, (1974 to date) and PsycINFO (1967 to date). We also searched international trial registries and contacted pharmaceutical companies and experts in the field.
Selection criteria: We included RCTs of women with depression with onset up to six months postpartum that compared antidepressant treatment (alone or in combination with another treatment) with any other treatment, placebo or treatment as usual.
Data collection and analysis: Two review authors independently extracted data from the trial reports. We requested missing information from investigators wherever possible. We sought data to allow an intention-to-treat analysis. Random effects meta-analyses were conducted to pool data where sufficient comparable studies were identified.
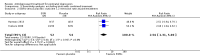
Main results: We included six trials with 596 participants in this review. All studies had a randomised controlled parallel group design, with two conducted in the UK, three in the US and one in Israel. Meta-analyses were performed to pool data on response and remission from studies comparing antidepressants with placebo. No meta-analyses could be conducted for other comparisons due to the small number of trials identified.Four studies compared selective serotonin reuptake inhibitors (SSRIs) with placebo (two using sertraline, one using paroxetine and one using fluoxetine; 233 participants in total). In two of these studies both the experimental and placebo groups also received psychological therapy. Pooled risk ratios based on data from three of these studies (146 participants) showed that women randomised to SSRIs had higher rates of response and remission than those randomised to placebo (response: RR 1.43, 95% CI 1.01 to 2.03; remission: RR 1.79, 95% CI 1.08 to 2.98); the fourth study did not report data on response or remission.One study (254 participants) compared antidepressant treatment with treatment as usual (for the first four weeks) followed by listening visits. The study found significantly higher rates of improvement in the antidepressant group than treatment-as-usual group after the first four weeks, but no difference between antidepressants and listening visits at the later follow-up. In addition, one study comparing sertraline with nortriptyline (a tricyclic antidepressant) found no difference in effectiveness (109 participants).Side effects were experienced by a substantial proportion of women, but there was no evidence of a meaningful difference in the number of adverse effects between treatment arms in any study. There were very limited data on adverse effects experienced by breastfed infants, with no long-term follow-up. All but one of the studies were assessed as being at high or uncertain risk of attrition bias and selective outcome reporting. In particular, one of the placebo-controlled studies had over 50% drop-out.
Authors' conclusions: The evidence base for this review was very limited, with a small number of studies and little information on a number of important outcomes, particularly regarding potential effects on the child. Risk of bias, for example from high attrition rates, as well as low representativeness of participants (e.g. exclusion of women with severe or chronic depression in several trials) also limit the conclusions that can be drawn.Pooled estimates for response and remission found that SSRIs were significantly more effective than placebo for women with postnatal depression. However the quality of evidence contributing to this comparison was assessed as very low owing to the small sample size for this comparison (146 participants from three studies), the risk of bias in included studes and the inclusion of one study where all participants in both study arms additionally received psychological therapy. There was insufficient evidence to conclude whether, and for whom, antidepressant or psychological/psychosocial treatments are more effective, or whether some antidepressants are more effective or better tolerated than others. There is also inadequate evidence on whether the benefits of antidepressants persist beyond eight weeks or whether they have short- or long-term adverse effects on breastfeeding infants.Professionals treating women with severe depression in the postnatal period will need to draw on other evidence, including trials among general adult populations and observational studies of antidepressant safety when breastfeeding (although the potential for confounding in non-randomised studies must be considered). More RCTs are needed with larger sample sizes and longer follow-up, including assessment of the impact on the child and safety of breastfeeding. Further larger-scale trials comparing antidepressants with alternative treatment modalities are also required.
Conflict of interest statement
Louise M Howard is Chair of the National Institute for Health and Care Excellence (NICE) (update) guideline on antenatal and postnatal mental health. She is Chief Investigator of an NIHR Programme Grant for Applied Research on the effectiveness of perinatal mental health services (RP‐ RP‐DG‐1108‐10012) and has funding from an NIHR Research Professorship on maternal mental health, and a grant from Tommy's baby charity (with the support of a corporate social responsibility grant from Johnson & Johnson) on antipsychotics in pregnancy. Her work is also supported by the NIHR Mental Health Biomedical Research Centre at the South London and Maudsley NHS Foundation Trust and King's College London. The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Kylee Trevillion is project manager on an NIHR Programme Grant for Applied Research on the effectiveness of perinatal mental health services (RP‐ RP‐DG‐1108‐10012).
Emma Molyneaux is supported by a Medical Research Council (MRC) PhD Studentship and Tommy's baby charity.
There are no other declarations of interest.
Figures














Update of
-
Antidepressant drug treatment for postnatal depression.Cochrane Database Syst Rev. 2001;(2):CD002018. doi: 10.1002/14651858.CD002018. Cochrane Database Syst Rev. 2001. Update in: Cochrane Database Syst Rev. 2014 Sep 11;(9):CD002018. doi: 10.1002/14651858.CD002018.pub2. PMID: 11406023 Updated.
References
References to studies included in this review
Appleby 1997 {published data only}
-
- Appleby L. A controlled study of fluoxetine and cognitive‐behavioural counselling in the treatment of postnatal depression. Proceedings of the 9th Congress of the Association of European Psychiatrists; 1998 Sept 20‐24; Copenhagen, Denmark.
-
- Warner R, Appleby L, Whitton A, Faragher B. A controlled study of fluoxetine and cognitive behavioural counselling in the treatment of post‐natal depression. Proceedings of the Royal College of Psychiatrists Winter Meeting; 1997 Jan 21‐24; Cardiff, UK.
Bloch 2012 {published data only}
-
- Bloch M, Meiboom H, Lorberblat M, Aharonov I, Bluvshtein I, Ben‐Itzhak S, et al. Treatment of postpartum depression with psychotherapy and add‐on sertraline: a double‐blind, randomised, placebo‐controlled study. European Neuropsychopharmacology 2011;21(Suppl 3):S359‐60.
-
- Bloch M, Meiboom H, Lorberblatt M, Bluvstein I, Aharonov I, Schreiber S. The effect of sertraline add‐on to brief dynamic psychotherapy for the treatment of postpartum depression: a randomized, double‐blind, placebo‐controlled study. Journal of Clinical Psychiatry 2012;73(2):235‐41. - PubMed
Hantsoo 2013 {published data only}
-
- Epperson CN, McDougle CJ, Ward‐O'Brien D, Price LH. A controlled study of antidepressant treatment of postpartum depression. Proceedings of the 149th Annual Meeting of the American Psychiatric Association; 1996 May 4‐9; New York.
Sharp 2010 {published data only}
-
- Howard L, Chew‐Graham CA, Tylee A, Lewis G, Anderson I, Abel K, et al. The RESPOND trial: a randomized evaluation of antidepressants and support for women with postnatal depression. Archives of Women's Mental Health 2011;14(Suppl 1).
-
- Sharp D J, Chew‐Graham C A, Tylee A, Lewis G, Howard L, Anderson I, et al. A pragmatic randomised controlled trial to compare antidepressants with a community‐based psychosocial intervention for the treatment of women with postnatal depression: the RESPOND trial. Health Technology Assessment 2010;14(43):1‐181. - PubMed
Wisner 2006 {published data only}
-
- Logsdon MC, Wisner K, Hanusa BH, Philips A. Role functioning and symptom remission in women with postpartum depression after antidepressant treatment. Archives of Psychiatric Nursing 2003;17(6):276‐83. - PubMed
-
- Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DK, et al. Postpartum depression: a randomized trial of sertraline versus nortriptyline. Journal of Clinical Psychopharmacology 2006;26(4):353‐60. - PubMed
Yonkers 2008 {published data only}
-
- Heath AC, Yonkers KA, Rush AJ. Paroxetine in the treatment of postpartum depression. Proceedings of the 153rd Annual Meeting of the American Psychiatric Association; 2000 May 13‐8; Chicago, IL.
-
- Heath AC, Yonkers KA, Rush AJ. Paroxetine in the treatment of postpartum depression. Proceedings of the 155th Annual Meeting of the American Psychiatric Association; 2002 May 18‐23; Philadelphia, PA.
References to studies excluded from this review
Bennett 2001 {published data only}
-
- Bennett P. The costs and benefits of treatment of post‐natal depression in the community. National Research Register 2001.
-
- Honey KL, Bennett P, Morgan M. A brief psycho‐educational group intervention for postnatal depression. British Journal of Clinical Psychology 2002;41:405‐9. - PubMed
Misri 2004 {published data only}
-
- Misri S, Reebye P, Corral M, Milis L. The use of paroxetine and cognitive‐behavioral therapy in postpartum depression and anxiety: a randomized controlled trial. Journal of Clinical Psychiatry 2004;65(9):1236‐41. - PubMed
-
- Misri S, Reebye P, Milis L, Shah S. The impact of treatment intervention on parenting stress in postpartum depressed mothers: a prospective study. American Journal of Orthopsychiatry 2006;76(1):115‐9. - PubMed
Rojas 2007 {published data only}
-
- Rojas G, Fritsch R, Solis J, Jadresic E, Castillo C, González M, et al. Treatment of postnatal depression in low‐income mothers in primary‐care clinics in Santiago, Chile: a randomised controlled trial. Lancet 2007;370(9599):1629‐37. - PubMed
-
- Zayas LH. Six‐month multicomponent intervention improves postnatal depression in low‐income settings. Evidence‐Based Mental Health 2008;11(3):80. - PubMed
Stein 2012 {published data only}
Suri 2005 {published data only}
-
- Suri R, Burt VK, Altshuler LL. Nefazodone for the treatment of postpartum depression. Archives of Women's Mental Health 2005;8(1):55‐6. - PubMed
Yu 2006 {published data only}
-
- Yu H, Liu C, Wang X. The therapeutic effects of paroxetine addition of psychological intervention on post‐partum depression. Progress in Obstetrics and Gynecology 2006;15(4):289‐91.
Zhao 2006 {published data only}
-
- Zhao X, Lin H. Study on postpartum depression treated with combination of fluoxetine and Chaihu Shugan powder. Liaoning Journal of Traditional Chinese Medicine 2006;33(5):586‐7.
References to studies awaiting assessment
NCT00744328 {unpublished data only}
-
- Wisner KL. Postpartum Depression: Transdermal Estradiol Versus Sertraline (E2 SERT). http://clinicaltrials.gov/show/NCT00744328 2014 (accessed 04 August 2014).
-
- Wisner KL. Should estradiol be prescribed for postpartum depression? [conference abstract]. Biological Psychiatry 2014;9(Suppl 1):299S.
NCT02122393 {unpublished data only}
-
- Milgrom J. A Randomised Trial of Sertraline, Cognitive Behaviour Therapy & Combined Therapy for Postnatal Depression. http://www.clinicaltrials.gov/ct2/show/NCT02122393 2014 (accessed 04 August 2014).
References to ongoing studies
NCT00602355 {unpublished data only}
-
- Zlotnick C, Stuart S. Placebo controlled trial of sertraline and interpersonal psychotherapy for postpartum depression. http://clinicaltrials.gov/ct2/show/NCT00602355 2008 (accessed 27 July 2014). - PubMed
Additional references
APA 1999
-
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington, DC: American Psychiatric Association, 1999.
APA 2013
-
- American Psychiatric Association. DSM 5. American Psychiatric Association, 2013.
Arroll 2005
Ban 2012
Bayley 2006
-
- Bayley N. Bayley Scales of Infant and Toddler Development® 3rd Edition (Bayley‐III®). San Antonio, TX: The Psychological Corporation, 2006.
Berle 2011
Berton 2006
-
- Berton O, Nestler EJ. New approaches to antidepressant drug discovery: beyond monoamines. Nature Reviews Neuroscience 2006;7:137‐51. - PubMed
Bland 2000
Centre for Research and Dissemination 2009
-
- Centre for Research and Dissemination. Systematic reviews: CRD's guidance for undertaking systematic reviews in healthcare. York, UK: University of York, 2009.
Cohen 1997
-
- Cohen S, Doyle WJ, Skoner DP, Rabin BS, Gwaltney JM Jr. Social ties and susceptibility to the common cold. JAMA 1997;277:1940‐4. - PubMed
Cooper 1995
-
- Cooper PJ, Murray L. Course and recurrence of postnatal depression. Evidence for the specificity of the diagnostic concept. British Journal of Psychiatry 1995;166:191‐5. - PubMed
Cox 1987
-
- Cox J, Holden JM, Sagovsky R. Detection of postnatal depression: development of the 10‐item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry 1987;150:782‐6. - PubMed
Cox 1993
-
- Cox JL, Murray D, Chapman G. A controlled study of the onset, duration and prevalence of postnatal depression. British Journal of Psychiatry 1993;163:27‐31. - PubMed
Crittenden 1988
-
- Crittenden PM. Relationships at Risk. In: Belsky J, Nezworski T editor(s). Clinical implications of attachment. Hillsdale, NJ: Lawrence Erlbaum Association, 1988:136‐74.
De Crescenzo 2014
-
- Crescenzo F, Perelli F, Armando M, Vicatri S. Selective serotonin reuptake inhibitors (SSRIs) for post‐partum depression (PPD): a systematic review of randomized clinical trials. Journal of Affective Disorders 2014;152:39‐44. - PubMed
Dennis 2013
Eberhard‐Gran 2006
-
- Eberhard‐Gran M, Esklid A, Opjordsmoen S. Use of psychotropic medications in treating mood disorders during lactation. Practical recommendations. CNS Drugs 2006;20:187‐98. - PubMed
Elbourne 2002
-
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. - PubMed
Endicott 1976
-
- Endicott J, Spitzer RL, Fleiss JL, Cohen J. The Global Assessment Scale: a procedure for measuring overall severity of psychiatric disturbance. Archives of General Psychiatry 1976;33(6):766. - PubMed
Figueiredo 2007
-
- Figueiredo B, Pacheco A, Costa R. Depression during pregnancy and the postpartum period in adolescent and adult Portuguese mothers. Archives of Women's Mental Health 2007;10(3):103‐9. - PubMed
Fisher 2012
Fournier 2010
Gavin 2005
-
- Gavin NI, Gaynes BN, Lohr KN, Meltzer‐Brody S, Gartlehner G, Swinson T. Perinatal depression: a systematic review of prevalence and incidence. Obstetrics & Gynecology 2005;106(5, Part 1):1071‐83. - PubMed
Gidaud‐Wallston 1978
-
- Gibaud‐Wallston J, Wandersman LP. Development and utility of the Parneting Sense of Competence Scale. Proceedings of the American Psychological Association; 1978 Aug 28 ‐ Sept 1; Toronto, Canada.
Goodman 2004
-
- Goodman JH. Postpartum depression beyond the early postpartum period. Journal of Obstetric, Gynecologic, & Neonatal Nursing 2004;33(4):410‐20. - PubMed
Gordon 2013
-
- Gordon A, Mikocka‐Walus A, Grzeskowiak LE, Jayaasekara RS. Antidepressants for depression during pregnancy. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD010710] - DOI
Henshaw 2003
-
- Henshaw C. Mood disturbance in the early puerperium: a review. Archives of Women’s Mental Health 2003;6(2):s33‐42. - PubMed
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Johnson 2008
-
- Johnson S, Dieter W, Marlow N. Developmental assessment of preterm infants at 2 years: validity of parent reports. Developmental Medicine and Child Neurology 2008;50(1):58‐62. - PubMed
Kaufman Kantor 2004
-
- Kaufman Kantor G, Holt MK, Mebert CJ, Straus MA, Drach KM, Ricci LR, et al. Development and preliminary psychometric properties of the Multidimensional Neglectful Behavior Scale‐Child Report. Child Maltreatment 2004;9(5):409‐28. - PubMed
Kendell 1987
-
- Kendell RE, Chalmers L, Platz C. The epidemiology of puerperal psychoses. British Journal of Psychiatry 1987;150:662‐73. - PubMed
Kirsch 2008
Letourneau 2012
-
- Letourneau NL, Dennis C‐L, Benzies K, Duffett‐Leger L, Stewart M, Tryphonopoulos PD, et al. Postpartum depression is a family affair: addressing the impact on mothers, fathers, and children. Issues in Mental Health Nursing 2012;33(7):445‐57. - PubMed
Ma 2014
-
- Ma Y. Neuropsychological mechanism underlying antidepressant effect: a systematic meta‐analysis. Molecular Psychiatry 2014 Mar 25 [Epub ahead of print]. - PubMed
Munk‐Olsen 2006
-
- Munk‐Olsen T, Laursen TM, Pedersen CB, Mors O, Mortensen PB. New parents and mental disorders. JAMA 2006;296(21):2582‐9. - PubMed
Murray 1992
-
- Murray L. The impact of postnatal depression on infant development. Journal of Child Psychology and Psychiatry 1992;33(3):543‐61. - PubMed
NICE 2007
-
- National Institute for Health and Clinical Excellence. Antenatal and Postnatal Mental Health: Clinical Management and Service Guidance. CG45. Vol. 45, National Institute for Health and Clinical Excellence, 2007. - PubMed
Norton 1983
-
- Norton R. Measuring marital quality: a critical look at the dependent variable. Journal of Marriage and Family 1983;45:141‐51.
Nylen 2013
-
- Nylen KJ, Williamson JA, O'Hara MW, Watson D, Engeldinger J. Validity of somatic symptoms as indicators of depression in pregnancy. Archives of Women's Mental Health 2013;16(3):1‐8. - PubMed
Pringle 2011
-
- Pringle A, Browning M, Cowen PJ, Harmer CJ. A cognitive neuropsychological model of antidepressant drug action. Progress in Neuro‐Psychopharmacology and Biological Psychiatry 2011;35(7):1586‐92. - PubMed
RevMan 2012 [Computer program]
-
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Schroeder 2006
-
- Schroeder K, Fahey T, Hay AD, Montgomery A, Peters TJ. Adherence to antihypertensive medication assessed by self‐report was associated with electronic monitoring compliance. Journal of Clinical Epidemiology 2006;59:650‐1. - PubMed
Spitzer 1978
-
- Spitzer RL, Endicott J, Robins E. Research diagnostic criteria: rationale and reliability. Archives of General Psychiatry 1978;35(6):773‐82. - PubMed
Ware 1992
-
- Ware JE, Sherbourne CD. The MOS 36‐item Short‐Form Health Survey (SF‐36): I. Conceptual framework and item selection. Medical Care 1992;30:473‐83. - PubMed
Wechsler 1974
-
- Wechsler D. Wechsler Intelligence Scale for Children ‐ Revised. New York, NY: Psychological Corp, 1974.
WHO 1992
-
- World Health Organization. The ICD‐10 Classification of Mental and Behavioural Disorders. Geneva: World Health Organization, 1992.
WHO 2004
-
- World Health Organization. ICD‐10: International Statistical Classification of Diseases and Related Health Problems. World Health Organization, 2004.
Wisner 2004
-
- Wisner KL, Perel JM, Peindl KS, Hanusa BH. Timing of depression recurrence in the first year after birth. Journal of Affective Disorders 2004;78(3):249‐52. - PubMed
Wisner 2013
Xia 2009
-
- Xia J, Adams C, Bhagat N, Bhagat V, Bhoopathi P, El‐Sayeh H, et al. Losing participants before the trial ends erodes credibility of findings. Psychiatric Bulletin 2009;33(7):254‐7.
Zimmerman 2013
-
- Zimmerman M, Martinez JH, Young D, Chelminski I, Dalrymple K. Severity classification on the Hamilton depression rating scale. Journal of Affective Disorders 2013;150:384‐388. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

