Efficacy and safety of abatacept for patients with Sjögren's syndrome associated with rheumatoid arthritis: rheumatoid arthritis with orencia trial toward Sjögren's syndrome Endocrinopathy (ROSE) trial-an open-label, one-year, prospective study-Interim analysis of 32 patients for 24 weeks
- PMID: 25211401
- PMCID: PMC4389760
- DOI: 10.3109/14397595.2014.951144
Efficacy and safety of abatacept for patients with Sjögren's syndrome associated with rheumatoid arthritis: rheumatoid arthritis with orencia trial toward Sjögren's syndrome Endocrinopathy (ROSE) trial-an open-label, one-year, prospective study-Interim analysis of 32 patients for 24 weeks
Abstract
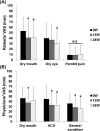
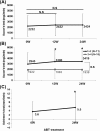
Abstract Objective. To assess the efficacy and safety of abatacept for secondary Sjögren's syndrome (SS) associated with rheumatoid arthritis (RA). Methods. The primary endpoint of this 1-year, open-labeled, prospective, observational multicenter study of RA-associated secondary SS was the rate of SDAI remission at 52 weeks after initiation of abatacept therapy. The secondary endpoints included that of Saxson's test and Schirmer's test. Adverse events during the study period were also analyzed. Results. Thirty-two patients (all females) were enrolled in this study. Interim analysis at 24 weeks included assessment of efficacy (n = 31) and safety (n = 32). The mean SDAI decreased from 19.8 ± 11.0 (± SD) at baseline to 9.9 ± 9.9 at 24 weeks (P < 0.05). Patients with clinical remission, as assessed by SDAI, increased from 0 patient (0 week) to 8 patients (25.8%) at 24 weeks. Saliva volume (assessed by Saxson's test) increased slightly from 2232 ± 1908 (0 week) to 2424 ± 2004 (24 weeks) mg/2 min (n = 29). In 11 patients with Greenspan grading 1/2 of labial salivary glands biopsy, saliva volume increased from 2945 ± 2090 (0 week) to 3419 ± 2121 (24 weeks) mg/2 min (P < 0.05). Schirmer's test for tear volume showed increase from 3.6 ± 4.6 (0 week) to 5.5 ± 7.1 (24 weeks) mm/5 min (n = 25; P < 0.05). Five adverse events occurred in five of 32 patients (15.6%), and three of these events were infections. Conclusion. Abatacept seems to be effective for both RA and RA-related secondary SS.
Keywords: Abatacept; Rheumatoid arthritis; Sjögren's syndrome.
Figures




Similar articles
-
New pharmacological strategies in rheumatic diseases.J Med Life. 2016 Jul-Sep;9(3):227-234. J Med Life. 2016. PMID: 27974925 Free PMC article. Review.
-
Effectiveness of abatacept for patients with Sjögren's syndrome associated with rheumatoid arthritis. An open label, multicenter, one-year, prospective study: ROSE (Rheumatoid Arthritis with Orencia Trial toward Sjögren's syndrome Endocrinopathy) trial.Mod Rheumatol. 2016 Nov;26(6):891-899. doi: 10.3109/14397595.2016.1158773. Epub 2016 Jul 26. Mod Rheumatol. 2016. PMID: 27459020 Clinical Trial.
-
Abatacept ameliorates both glandular and extraglandular involvements in patients with Sjögren's syndrome associated with rheumatoid arthritis: Findings from an open-label, multicentre, 1-year, prospective study: The ROSE (Rheumatoid Arthritis with Orencia Trial Toward Sjögren's Syndrome Endocrinopathy) and ROSE II trials.Mod Rheumatol. 2023 Jan 3;33(1):160-168. doi: 10.1093/mr/roac011. Mod Rheumatol. 2023. PMID: 35134994
-
Diffusion-weighted magnetic resonance imaging of parotid glands before and after abatacept therapy in patients with Sjögren's syndrome associated with rheumatoid arthritis: Utility to evaluate and predict response to treatment.Mod Rheumatol. 2018 Mar;28(2):300-307. doi: 10.1080/14397595.2017.1349234. Epub 2017 Jul 19. Mod Rheumatol. 2018. PMID: 28722506
-
World Workshop on Oral Medicine VII: Immunobiologics for salivary gland disease in Sjögren's syndrome: A systematic review.Oral Dis. 2019 Jun;25 Suppl 1(Suppl 1):102-110. doi: 10.1111/odi.13062. Oral Dis. 2019. PMID: 31140693 Free PMC article.
Cited by
-
Activity of rheumatoid arthritis correlates with oral inflammatory burden.Rheumatol Int. 2018 Sep;38(9):1661-1669. doi: 10.1007/s00296-018-4108-z. Epub 2018 Jul 24. Rheumatol Int. 2018. PMID: 30043237
-
Natural and iatrogenic ocular manifestations of rheumatoid arthritis: a systematic review.Int Ophthalmol. 2022 Feb;42(2):689-711. doi: 10.1007/s10792-021-02058-8. Epub 2021 Nov 21. Int Ophthalmol. 2022. PMID: 34802085 Free PMC article.
-
Composition and regulation of the immune microenvironment of salivary gland in Sjögren's syndrome.Front Immunol. 2022 Sep 13;13:967304. doi: 10.3389/fimmu.2022.967304. eCollection 2022. Front Immunol. 2022. PMID: 36177010 Free PMC article.
-
RORγt antagonist suppresses M3 muscarinic acetylcholine receptor-induced Sjögren's syndrome-like sialadenitis.Clin Exp Immunol. 2017 Feb;187(2):213-224. doi: 10.1111/cei.12868. Epub 2016 Oct 11. Clin Exp Immunol. 2017. PMID: 27643385 Free PMC article.
-
New pharmacological strategies in rheumatic diseases.J Med Life. 2016 Jul-Sep;9(3):227-234. J Med Life. 2016. PMID: 27974925 Free PMC article. Review.
References
-
- Tsuboi H, Asashima H, Takai C, Hagiwara S, Hagiya C, Yokosawa M, et al. Primary and secondary surveys on epidemiology of Sjogren's syndrome in Japan. Mod Rheumatol. 2014;24(3):464–70. - PubMed
-
- Ramos-Casals M, Brito-Zerón P, Sisó-Almirall A, Bosch X, Tzioufas AG. Topical and systemic medications for the treatment of primary Sjögren's syndrome. Nat Rev Rheumatol. 2012;8(7):399–411. - PubMed
-
- Meijer JM, Meiners PM, Vissink A, Spijkervet FK, Abdulahad W, Kamminga N, et al. Effectiveness of rituximab treatment in primary Sjögren's syndrome: a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2010;62(4):960–8. - PubMed
-
- Dass S, Bowman SJ, Vital EM, Ikeda K, Pease CT, Hamburger J, et al. Reduction of fatigue in Sjögren syndrome with rituximab: results of a randomised, double-blind, placebo-controlled pilot study. Ann Rheum Dis. 2008;67(11):1541–4. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

