The role of genetics in estrogen responses: a critical piece of an intricate puzzle
- PMID: 25212221
- PMCID: PMC4232287
- DOI: 10.1096/fj.14-260307
The role of genetics in estrogen responses: a critical piece of an intricate puzzle
Abstract
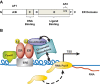
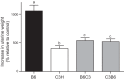
The estrogens are female sex hormones that are involved in a variety of physiological processes, including reproductive development and function, wound healing, and bone growth. They are mainly known for their roles in reproductive tissues--specifically, 17β-estradiol (E2), the primary estrogen, which is secreted by the ovaries and induces cellular proliferation and growth of the uterus and mammary glands. In addition to the role of estrogens in promoting tissue growth and development during normal physiological states, they have a well-established role in determining susceptibility to disease, particularly cancer, in reproductive tissues. The responsiveness of various tissues to estrogen is genetically controlled, with marked quantitative variation observed across multiple species, including humans. This variation presents both researchers and clinicians with a veritable physiological puzzle, the pieces of which--many of them unknown--are complex and difficult to fit together. Although genetics is known to play a major role in determining sensitivity to estrogens, there are other factors, including parent of origin and the maternal environment, that are intimately linked to heritable phenotypes but do not represent genotype, per se. The objectives of this review article were to summarize the current knowledge of the role of genotype, and uterine and neonatal environments, in phenotypic variation in the response to estrogens; to discuss recent findings and the potential mechanisms involved; and to highlight exciting research opportunities for the future.
Keywords: genotype; reproductive tissue.
© FASEB.
Figures

References
-
- Katzenellenbogen B. S., Bhakoo H. S., Ferguson E. R., Lan N. C., Tatee T., Tsai T. S., Katzenellenbogen J. A. (1979) Estrogen and antiestrogen action in reproductive tissues and tumors. Recent Prog. Horm. Res. 35, 259–300 - PubMed
-
- Clark J. H., Mani S. K. (1994) Actions of ovarian steroid hormones. In The Physiology of Reproduction, Vol. 1 (Knobil E., Neill J., eds) pp. 1011–1059, Raven Press, New York
-
- Perez M. C., Furth E. E., Matzumura P. D., Lyttle C. R. (1996) Role of eosinophils in uterine responses to estrogen. Biol. Reprod. 54, 249–254 - PubMed
-
- Drasher M. L. (1952) Strain differences in the response of the mouse uterus to estrogens. J. Hered. 46, 190–192
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

