BAG3 facilitates the clearance of endogenous tau in primary neurons
- PMID: 25212465
- PMCID: PMC4268232
- DOI: 10.1016/j.neurobiolaging.2014.08.012
BAG3 facilitates the clearance of endogenous tau in primary neurons
Abstract
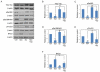
Tau is a microtubule associated protein that is found primarily in neurons, and in pathologic conditions, such as Alzheimer's disease (AD) it accumulates and contributes to the disease process. Because tau plays a fundamental role in the pathogenesis of AD and other tauopathies, and in AD mouse models reducing tau levels improves outcomes, approaches that facilitate tau clearance are being considered as therapeutic strategies. However, fundamental to the development of such interventions is a clearer understanding of the mechanisms that regulate tau clearance. Here, we report a novel mechanism of tau degradation mediated by the co-chaperone BAG3. BAG3 has been shown to be an essential component of a complex that targets substrates to the autophagy pathway for degradation. In rat primary neurons, activation of autophagy by inhibition of proteasome activity or treatment with trehalose resulted in significant decreases in tau and phospho-tau levels. These treatments also induced an upregulation of BAG3. Proteasome inhibition activated JNK, which was responsible for the upregulation of BAG3 and increased tau clearance. Inhibiting JNK or knocking down BAG3 blocked the proteasome inhibition-induced decreases in tau. Further, BAG3 overexpression alone resulted in significant decreases in tau and phospho-tau levels in neurons. These results indicate that BAG3 plays a critical role in regulating the levels of tau in neurons, and interventions that increase BAG3 levels could provide a therapeutic approach in the treatment of AD.
Keywords: Alzheimer's disease; Autophagy; BAG3; Co-chaperone; JNK; Tau.
Copyright © 2015 Elsevier Inc. All rights reserved.
Figures






References
-
- Behl C. BAG3 and friends: co-chaperones in selective autophagy during aging and disease. Autophagy. 2011;7(7):795–8. doi:10.4161/auto.7.7.15844. - PubMed
-
- Boland B, Kumar A, Lee S, Platt FM, Wegiel J, Yu WH, Nixon RA. Autophagy induction and autophagosome clearance in neurons: relationship to autophagic pathology in Alzheimer's disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28(27):6926–37. doi:10.1523/JNEUROSCI.0800-08.2008. - PMC - PubMed
-
- Carra S, Seguin SJ, Lambert H, Landry J. HspB8 chaperone activity toward poly(Q)-containing proteins depends on its association with Bag3, a stimulator of macroautophagy. The Journal of biological chemistry. 2008;283(3):1437–44. doi:10.1074/jbc.M706304200. - PubMed
-
- Carrettiero DC, Hernandez I, Neveu P, Papagiannakopoulos T, Kosik KS. The cochaperone BAG2 sweeps paired helical filament-insoluble tau from the microtubule. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29(7):2151–61. doi:10.1523/JNEUROSCI.4660-08.2009. - PMC - PubMed
-
- Chondrogianni N, Gonos ES. Proteasome dysfunction in mammalian aging: steps and factors involved. Experimental gerontology. 2005;40(12):931–8. doi:10.1016/j.exger.2005.09.004. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

