Influence of intrathecal delivery of bone marrow-derived mesenchymal stem cells on spinal inflammation and pain hypersensitivity in a rat model of peripheral nerve injury
- PMID: 25212534
- PMCID: PMC4172959
- DOI: 10.1186/s12974-014-0157-8
Influence of intrathecal delivery of bone marrow-derived mesenchymal stem cells on spinal inflammation and pain hypersensitivity in a rat model of peripheral nerve injury
Abstract
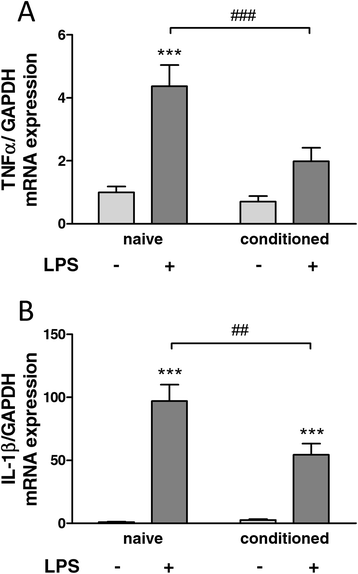
Background: Multipotent mesenchymal stem (stromal) cells (MSCs) have been credited with immunomodulative properties, supporting beneficial outcomes when transplanted into a variety of disease models involving inflammation. Potential mechanisms include the secretion of paracrine factors and the establishment of a neurotrophic microenvironment. To test the hypothesis that MSCs release soluble mediators that can attenuate local inflammation, we here analysed the influence of MSCs on the activation of microglia cells, as well as on inflammatory parameters and pain behaviour in a surgical rat model of neuropathic pain.
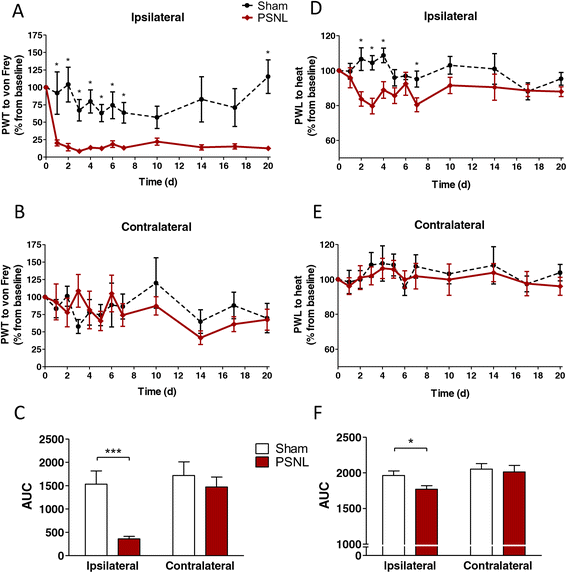
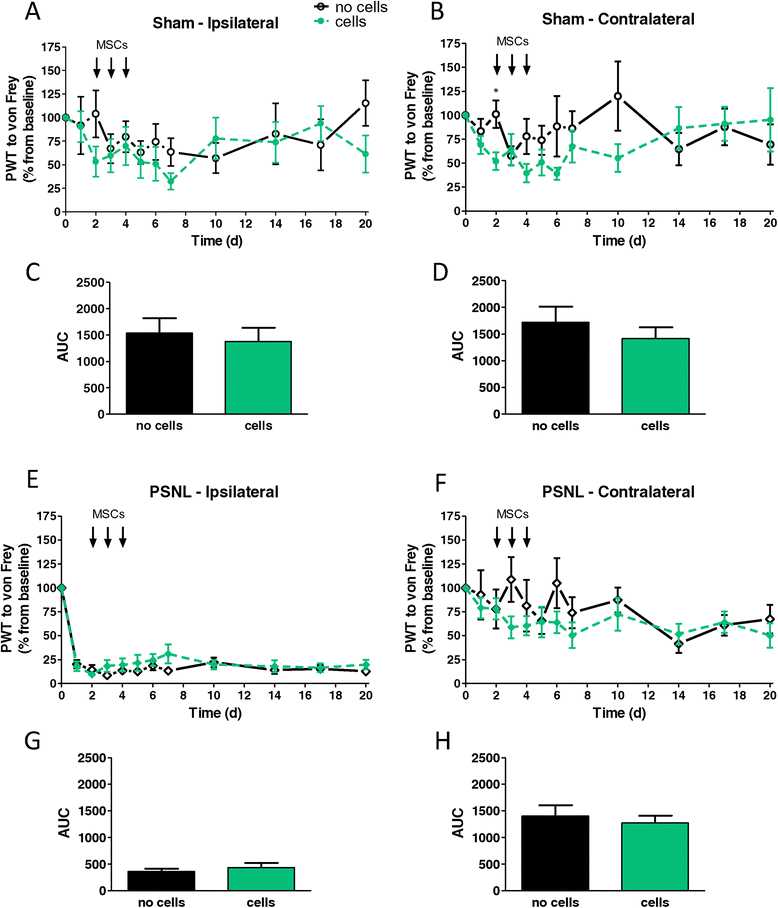
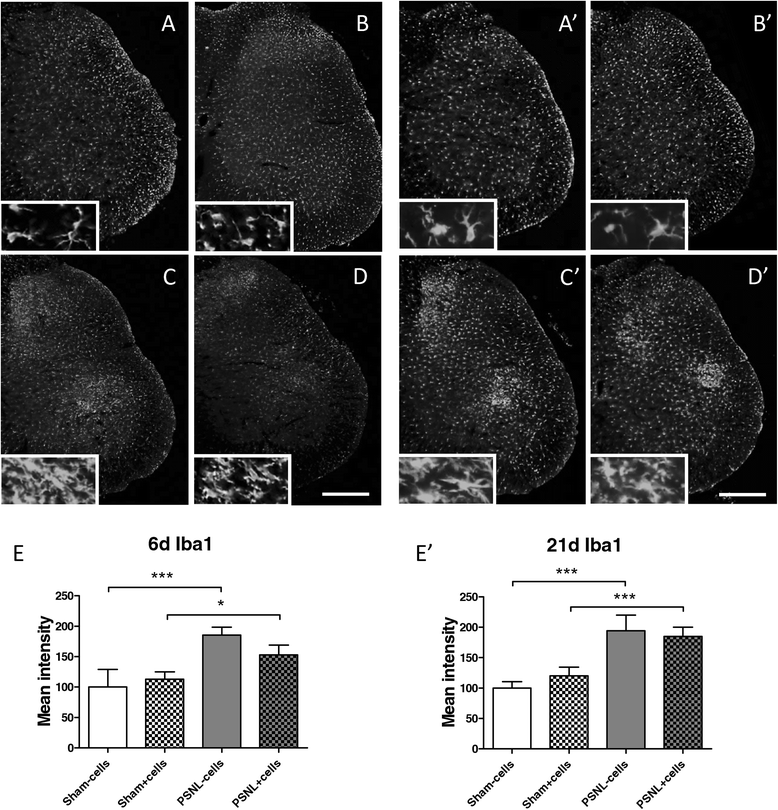
Methods: We focussed on an experimental model of partial sciatic nerve ligation (PSNL), characterised by a rapid and persistent inflammation in the dorsal lumbar spinal cord where sensory inputs from the sciatic nerve are processed. Via indwelling intrathecal catheters, MSCs were repetitively grafted into the intrathecal lumbar space. Animals were evaluated for mechanical and thermal hypersensitivity over a period of 21 days after PSNL. Afterwards, spinal cords were processed for immunohistochemical analysis of the microglial marker ionized calcium-binding adapter molecule 1 (Iba1) and quantification of inflammatory markers in ipsilateral dorsal horns. We hypothesised that injections on postsurgical days 2 to 4 would interfere with microglial activation, leading to a reduced production of pro-inflammatory cytokines and amelioration of pain behaviour.
Results: PSNL-induced mechanical allodynia or heat hyperalgesia were not influenced by MSC transplantation, and spinal cord inflammatory processes remained largely unaffected. Indeed, the early microglial response to PSNL characterised by increased Iba1 expression in the lumbar dorsal horn was not significantly altered and cytokine levels in the spinal cord at 21 days after surgery were similar to those found in vehicle-injected animals. Grafted MSCs were detected close to the pia mater, but were absent within the spinal cord parenchyma.
Conclusions: We conclude that intrathecal administration is not an appropriate route to deliver cells for treatment of acute spinal cord inflammation as it leads to entrapment of grafted cells within the pia mater. We propose that the early inflammatory response triggered by PSNL in the lumbar spinal cord failed to effectively recruit MSCs or was insufficient to disturb the tissue integrity so as to allow MSCs to penetrate the spinal cord parenchyma.
Figures




Similar articles
-
Enhanced neuroinflammation and pain hypersensitivity after peripheral nerve injury in rats expressing mutated superoxide dismutase 1.J Neuroinflammation. 2011 Apr 13;8:33. doi: 10.1186/1742-2094-8-33. J Neuroinflammation. 2011. PMID: 21489258 Free PMC article.
-
Anti-inflammatory protein TSG-6 secreted by bone marrow mesenchymal stem cells attenuates neuropathic pain by inhibiting the TLR2/MyD88/NF-κB signaling pathway in spinal microglia.J Neuroinflammation. 2020 May 11;17(1):154. doi: 10.1186/s12974-020-1731-x. J Neuroinflammation. 2020. PMID: 32393298 Free PMC article.
-
Intravenous administration of human mesenchymal stem cells derived from adipose tissue and umbilical cord improves neuropathic pain via suppression of neuronal damage and anti-inflammatory actions in rats.PLoS One. 2022 Feb 14;17(2):e0262892. doi: 10.1371/journal.pone.0262892. eCollection 2022. PLoS One. 2022. PMID: 35157707 Free PMC article.
-
Mesenchymal Stem Cells Transplantation for Neuropathic Pain Induced By Peripheral Nerve Injury in Animal Models: A Systematic Review.Stem Cells Dev. 2020 Nov 15;29(22):1420-1428. doi: 10.1089/scd.2020.0131. Epub 2020 Oct 22. Stem Cells Dev. 2020. PMID: 32962522
-
Serious Adverse Events Have Not Been Reported with Spinal Intrathecal Injection of Mesenchymal Stem Cells: A Systematic Review.Curr Stem Cell Res Ther. 2023;18(6):829-833. doi: 10.2174/1574888X17666220817125324. Curr Stem Cell Res Ther. 2023. PMID: 35980065
Cited by
-
Study on the Mechanism of BMSCs in Regulating NF-κB Signal Pathway by Targeting miR-449a to Improve the Inflammatory Response to Peripheral Nerve Injury.J Musculoskelet Neuronal Interact. 2022 Dec 1;22(4):546-561. J Musculoskelet Neuronal Interact. 2022. PMID: 36458392 Free PMC article.
-
Human bone marrow-derived and umbilical cord-derived mesenchymal stem cells for alleviating neuropathic pain in a spinal cord injury model.Stem Cell Res Ther. 2016 Mar 8;7:36. doi: 10.1186/s13287-016-0295-2. Stem Cell Res Ther. 2016. PMID: 26957122 Free PMC article.
-
Cell therapy for neuropathic pain.Front Mol Neurosci. 2023 Feb 27;16:1119223. doi: 10.3389/fnmol.2023.1119223. eCollection 2023. Front Mol Neurosci. 2023. PMID: 36923653 Free PMC article. Review.
-
Inhibition of neuropathic hyperalgesia by intrathecal bone marrow stromal cells is associated with alteration of multiple soluble factors in cerebrospinal fluid.Exp Brain Res. 2017 Sep;235(9):2627-2638. doi: 10.1007/s00221-017-5000-x. Epub 2017 Jun 1. Exp Brain Res. 2017. PMID: 28573310 Free PMC article.
-
Optimization strategies for mesenchymal stem cell-based analgesia therapy: a promising therapy for pain management.Stem Cell Res Ther. 2024 Jul 18;15(1):211. doi: 10.1186/s13287-024-03828-8. Stem Cell Res Ther. 2024. PMID: 39020426 Free PMC article. Review.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources

