Assessing statistical competencies in clinical and translational science education: one size does not fit all
- PMID: 25212569
- PMCID: PMC4329089
- DOI: 10.1111/cts.12204
Assessing statistical competencies in clinical and translational science education: one size does not fit all
Abstract
Introduction: Statistics is an essential training component for a career in clinical and translational science (CTS). Given the increasing complexity of statistics, learners may have difficulty selecting appropriate courses. Our question was: what depth of statistical knowledge do different CTS learners require?
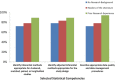
Methods: For three types of CTS learners (principal investigator, co-investigator, informed reader of the literature), each with different backgrounds in research (no previous research experience, reader of the research literature, previous research experience), 18 experts in biostatistics, epidemiology, and research design proposed levels for 21 statistical competencies.
Results: Statistical competencies were categorized as fundamental, intermediate, or specialized. CTS learners who intend to become independent principal investigators require more specialized training, while those intending to become informed consumers of the medical literature require more fundamental education. For most competencies, less training was proposed for those with more research background.
Discussion: When selecting statistical coursework, the learner's research background and career goal should guide the decision. Some statistical competencies are considered to be more important than others. Baseline knowledge assessments may help learners identify appropriate coursework.
Conclusion: Rather than one size fits all, tailoring education to baseline knowledge, learner background, and future goals increases learning potential while minimizing classroom time.
Keywords: assessment; research training; statistical competency.
© 2014 Wiley Periodicals, Inc.
Figures
References
-
- Altman DG, Goodman SN, Schroter S. How statistical expertise is used in medical research. JAMA. 2002; 287: 2817–2820. - PubMed
-
- Horton NJ, Switzer SS. Statistical methods in the journal. New Engl J Med. 2005; 353: 1977–1979. - PubMed
-
- Strasak AM, Zaman Q, Marinell G, Pfeiffer KP, Ulmer H. The use of statistics in medical research: a comparison of The New England Journal of Medicine and Nature Medicine . Am Stat 2007; 61: 47–55.
-
- Harris AHS, Reeder R, Hyun JK. Common statistical and research design problem in manuscripts submitted to high‐impact psychiatry journals: what editors and reviewers want authors to know. J Psychiat Res. 2009; 43: 1231–1234. - PubMed
-
- Mazumdar M, Banerjee S, Van Epps HL. Improved reporting of statistical design and analysis: guidelines, education, and editorial policies. Methods Mol Biol. 2010; 620: 563–598. - PubMed
Publication types
MeSH terms
Grants and funding
- UL1 TR000150/TR/NCATS NIH HHS/United States
- UL1 TR000165/TR/NCATS NIH HHS/United States
- UL1 TR000042/TR/NCATS NIH HHS/United States
- KL2 TR001118/TR/NCATS NIH HHS/United States
- UL1 TR000003/TR/NCATS NIH HHS/United States
- UL1 TR000135/TR/NCATS NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UL1 TR001417/TR/NCATS NIH HHS/United States
- UL1 TR000457/TR/NCATS NIH HHS/United States
- UL1 TR000371/TR/NCATS NIH HHS/United States
- P30 DK079626/DK/NIDDK NIH HHS/United States
- UL1 RR025767/RR/NCRR NIH HHS/United States
- UL1 TR001120/TR/NCATS NIH HHS/United States
- UL1 TR000077/TR/NCATS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials