Gammaretroviral pol sequences act in cis to direct polysome loading and NXF1/NXT-dependent protein production by gag-encoded RNA
- PMID: 25212909
- PMCID: PMC4174252
- DOI: 10.1186/s12977-014-0073-0
Gammaretroviral pol sequences act in cis to direct polysome loading and NXF1/NXT-dependent protein production by gag-encoded RNA
Abstract
Background: All retroviruses synthesize essential proteins via alternatively spliced mRNAs. Retrovirus genera, though, exploit different mechanisms to coordinate the synthesis of proteins from alternatively spliced mRNAs. The best studied of these retroviral, post-transcriptional effectors are the trans-acting Rev protein of lentiviruses and the cis-acting constitutive transport element (CTE) of the betaretrovirus Mason-Pfizer monkey virus (MPMV). How members of the gammaretrovirus genus translate protein from unspliced RNA has not been elucidated.
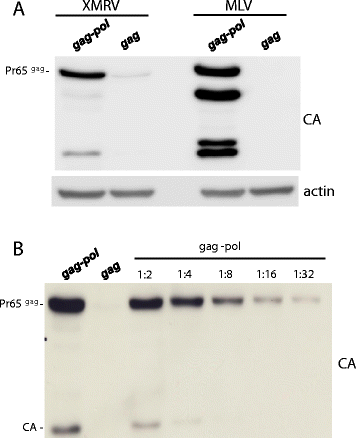
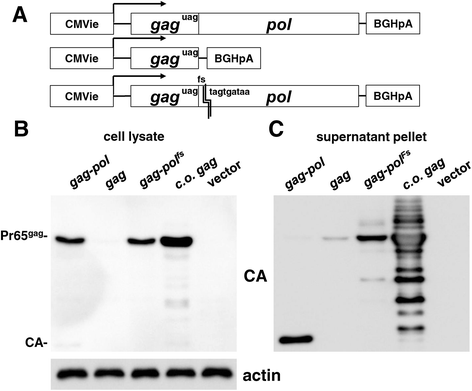
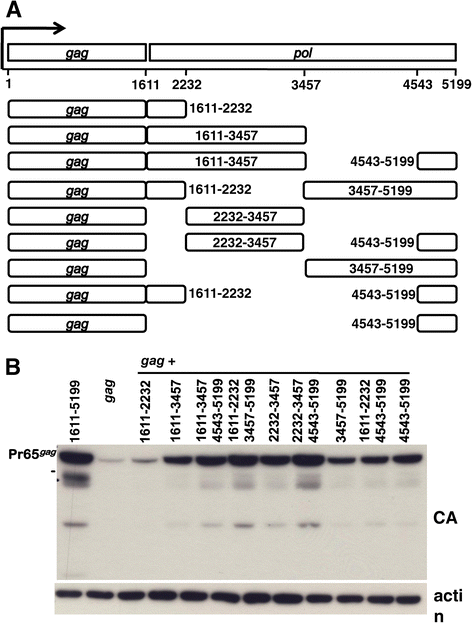
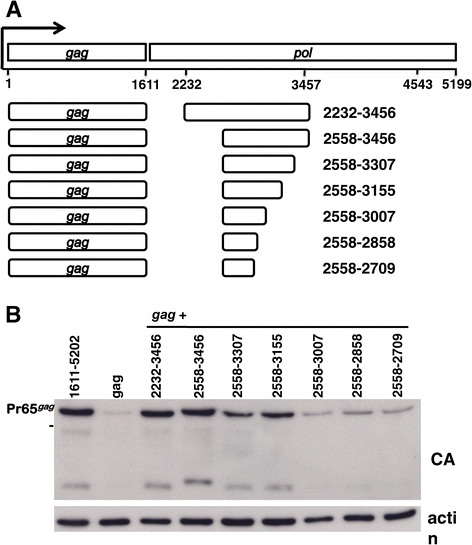
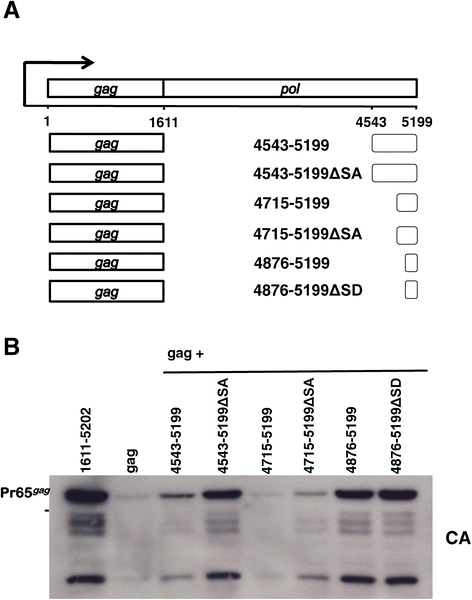
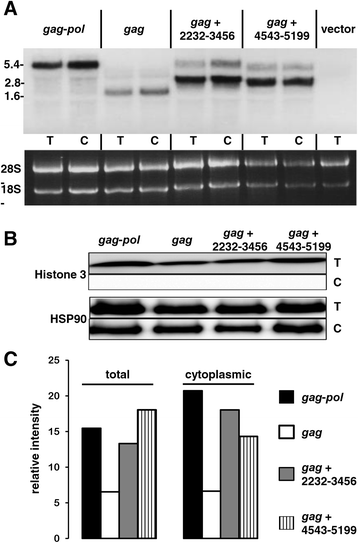
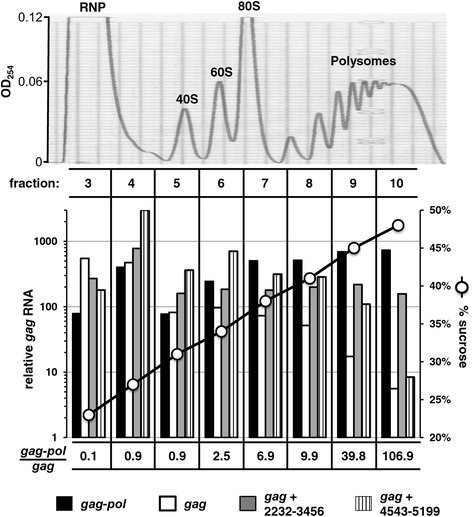
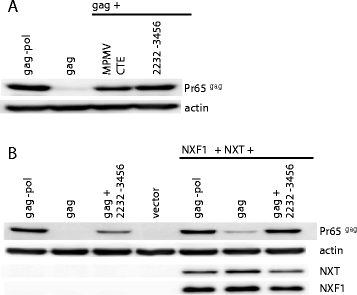
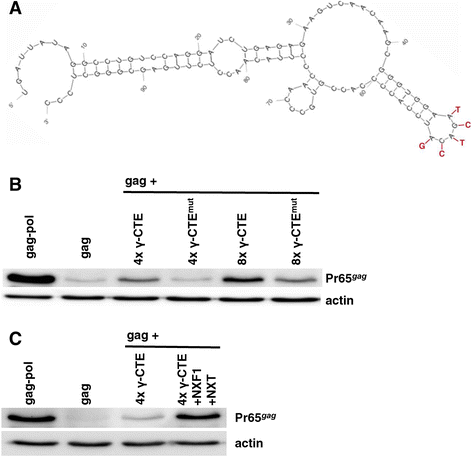
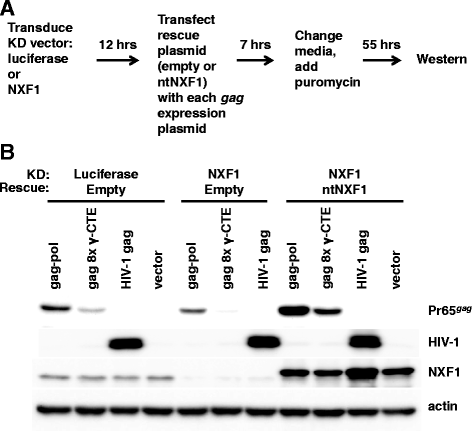
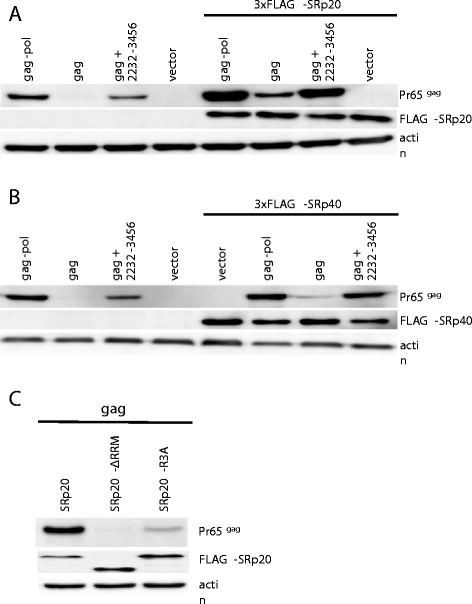
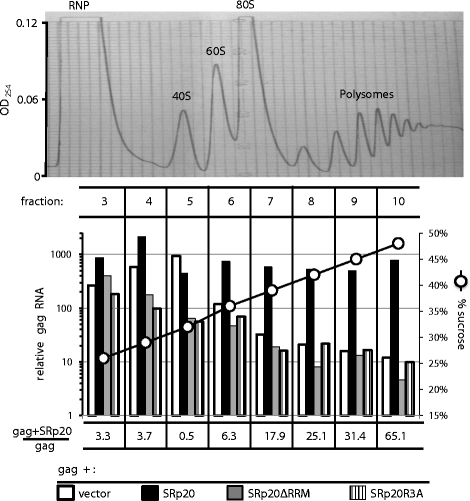
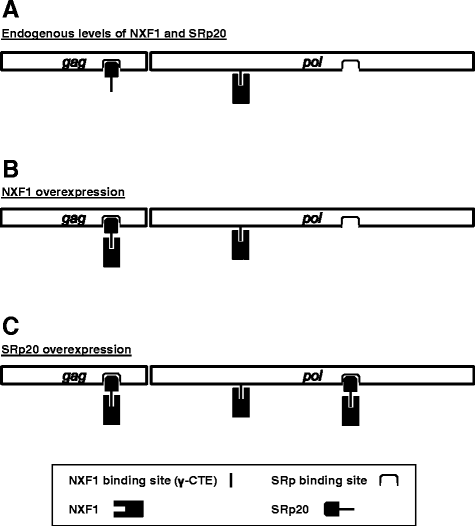
Results: The mechanism by which two gammaretroviruses, XMRV and MLV, synthesize the Gag polyprotein (Pr65Gag) from full-length, unspliced mRNA was investigated here. The yield of Pr65Gag from a gag-only expression plasmid was found to be at least 30-fold less than that from an otherwise isogenic gag-pol expression plasmid. A frameshift mutation disrupting the pol open reading frame within the gag-pol expression plasmid did not decrease Pr65Gag production and 398 silent nucleotide changes engineered into gag rendered Pr65Gag synthesis pol-independent. These results are consistent with pol-encoded RNA acting in cis to promote Pr65Gag translation. Two independently-acting pol fragments were identified by screening 17 pol deletion mutations. To determine the mechanism by which pol promoted Pr65Gag synthesis, gag RNA in total and cytoplasmic fractions was quantitated by northern blot and by RT-PCR. The pol sequences caused, maximally, three-fold increase in total or cytoplasmic gag mRNA. Instead, pol sequences increased gag mRNA association with polyribosomes ~100-fold, a magnitude sufficient to explain the increase in Pr65Gag translation efficiency. The MPMV CTE, an NXF1-binding element, substituted for pol in promoting Pr65Gag synthesis. A pol RNA stem-loop resembling the CTE promoted Pr65Gag synthesis. Over-expression of NXF1 and NXT, host factors that bind to the MPMV CTE, synergized with pol to promote gammaretroviral gag RNA loading onto polysomes and to increase Pr65Gag synthesis. Conversely, Gag polyprotein synthesis was decreased by NXF1 knockdown. Finally, overexpression of SRp20, a shuttling protein that binds to NXF1 and promotes NXF1 binding to RNA, also increased gag RNA loading onto polysomes and increased Pr65Gag synthesis.
Conclusion: These experiments demonstrate that gammaretroviral pol sequences act in cis to recruit NXF1 and SRp20 to promote polysome loading of gag RNA and, thereby license the synthesis of Pr65Gag from unspliced mRNA.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

