Fixed-dose combination of tafluprost and timolol in the treatment of open-angle glaucoma and ocular hypertension: comparison with other fixed-combination products
- PMID: 25213118
- PMCID: PMC4177040
- DOI: 10.1007/s12325-014-0151-7
Fixed-dose combination of tafluprost and timolol in the treatment of open-angle glaucoma and ocular hypertension: comparison with other fixed-combination products
Abstract
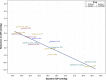
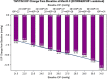
A new preservative-free fixed-dose combination of 0.0015% tafluprost, a prostaglandin F2α analog, and 0.5% timolol (TAF/TIM; Santen Oy, Tampere, Finland), a beta-adrenergic antagonist has recently been developed. The intraocular pressure (IOP) reduction with TAF/TIM in open-angle glaucoma and ocular hypertension is similar to that of other prostaglandin-timolol fixed-combination products. Patients with high IOP responded well to TAF/TIM with reductions of up to 40% (>13 mmHg) and beyond. Compared to previous controlled and double-masked clinical trials with DuoTrav(®) (Alcon, Fort Worth, USA) and Ganfort(®) (Allergan, Irvine, USA), TAF/TIM caused less superficial ocular side effects and less conjunctival hyperemia. Plausible explanations for the differences in side effects between the fixed-combination products are discussed.
Figures

Similar articles
-
A 6-month study comparing efficacy, safety, and tolerability of the preservative-free fixed combination of tafluprost 0.0015% and timolol 0.5% versus each of its individual preservative-free components.Adv Ther. 2014 Dec;31(12):1228-46. doi: 10.1007/s12325-014-0163-3. Epub 2014 Dec 2. Adv Ther. 2014. PMID: 25447269 Free PMC article. Clinical Trial.
-
Multicenter, Randomized, Controlled Study Comparing Tafluprost/Timolol Fixed Combination with Latanoprost/Timolol Fixed Combination in Primary Open-Angle Glaucoma and Ocular Hypertension.Adv Ther. 2018 Jun;35(6):796-808. doi: 10.1007/s12325-018-0718-9. Epub 2018 Jun 5. Adv Ther. 2018. PMID: 29873009 Clinical Trial.
-
Treatment of Open-Angle Glaucoma and Ocular Hypertension with Preservative-Free Tafluprost/Timolol Fixed-Dose Combination Therapy: The VISIONARY Study.Adv Ther. 2020 Apr;37(4):1436-1451. doi: 10.1007/s12325-020-01239-8. Epub 2020 Feb 18. Adv Ther. 2020. PMID: 32072493 Free PMC article.
-
Tafluprost/Timolol: A Review in Open-Angle Glaucoma or Ocular Hypertension.Drugs. 2015 Oct;75(15):1807-13. doi: 10.1007/s40265-015-0476-9. Drugs. 2015. PMID: 26431840 Review.
-
Efficacy and safety of tafluprost 0.0015% and timolol maleate 0.5% fixed combination in patients with ocular hypertension or open-angle glaucoma.Expert Opin Pharmacother. 2014 Oct;15(15):2255-62. doi: 10.1517/14656566.2014.955471. Epub 2014 Aug 29. Expert Opin Pharmacother. 2014. PMID: 25170534 Review.
Cited by
-
Changes in ocular signs and symptoms in patients switching from bimatoprost-timolol to tafluprost-timolol eye drops: an open-label phase IV study.BMJ Open. 2019 Apr 2;9(4):e024129. doi: 10.1136/bmjopen-2018-024129. BMJ Open. 2019. PMID: 30944129 Free PMC article. Clinical Trial.
-
Effectiveness and Safety of Switching to Fixed-Dose, Preservative-Free Tafluprost/Timolol in the Treatment of Open-Angle Glaucoma or Ocular Hypertension: A Real-World Study in Taiwan.Cureus. 2025 Jul 7;17(7):e87447. doi: 10.7759/cureus.87447. eCollection 2025 Jul. Cureus. 2025. PMID: 40772200 Free PMC article.
-
Evidence in Practice: A Review of Real-Life Studies and Clinical Experience with the Preservative-Free Tafluprost (0.0015%) and Timolol (0.5%) Fixed-Dose Combination.Clin Ophthalmol. 2024 Nov 8;18:3185-3196. doi: 10.2147/OPTH.S479852. eCollection 2024. Clin Ophthalmol. 2024. PMID: 39534090 Free PMC article. Review.
-
Improvement of corneal epithelial damage after switching from the concomitant use of brinzolamide and brimonidine to a brinzolamide/brimonidine fixed-dose combination.Jpn J Ophthalmol. 2024 Sep;68(5):556-561. doi: 10.1007/s10384-024-01088-w. Epub 2024 Jul 11. Jpn J Ophthalmol. 2024. PMID: 38990388
-
Daily Costs and Cost Effectiveness of Glaucoma Fixed Combinations in China.J Ophthalmol. 2020 Jul 30;2020:2406783. doi: 10.1155/2020/2406783. eCollection 2020. J Ophthalmol. 2020. PMID: 32850141 Free PMC article.
References
-
- Stjernschantz J. From PGF2α -isopropyl ester to latanoprost: a review of the development of Xalatan. Investig Ophthalmol Vis Sci. 2001;42:1134–1145. - PubMed
-
- Hellberg MR, McLaughlin MA, Sharif NA, et al. Identification and characterization of the ocular hypotensive efficacy of travoprost, a potent and selective FP prostaglandin receptor agonist, and AL-6598, a DP prostaglandin receptor agonist. Surv Ophthalmol. 2002;47(Suppl 1):S13–S33. doi: 10.1016/S0039-6257(02)00293-X. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

