Identification of unknown colorants in pre-Columbian textiles dyed with American cochineal (Dactylopius coccus Costa) using high-performance liquid chromatography and tandem mass spectrometry
- PMID: 25213214
- PMCID: PMC4305107
- DOI: 10.1007/s00216-014-8107-y
Identification of unknown colorants in pre-Columbian textiles dyed with American cochineal (Dactylopius coccus Costa) using high-performance liquid chromatography and tandem mass spectrometry
Abstract
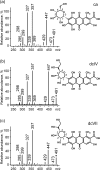
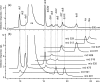
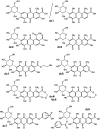
The present study concerns the identification of nine thus-far unknown derivatives of carminic acid extracted from pre-Columbian Peruvian textiles dyed with American cochineal-these derivatives are not found in commercially available preparations of the dye. These compounds probably represent a unique fingerprint of dyed textiles from this region, as they have never been reported to occur in other fabrics of historical value. They were separated by reversed-phase high-performance liquid chromatography (phenyl column) and detected using a UV/vis spectrophotometer and two tandem mass spectrometers. Peaks observed in chromatograms registered at 450 and 500 nm were further identified by ESI QqQ MS (mainly in the negative ion mode), supported by high-resolution ESI QIT/ToF MS data. The characteristic fragmentation pathways of isolated carminic acid and its derivatives provided additional information concerning lost neutrals and thus the functional groups and substituents present in the parent molecules. This information mainly related to multiple cleavages of the hexoside moiety (initially cross-ring cleavage), which are characteristic of C-glucosides (loss of 90, 120, and 148 Da). This is accompanied by the elimination of H2O as well as the further loss of 60 Da from the hexoside moiety. Moreover, other losses from the carbonyl groups (44 Da from CO2 loss, 62 Da from ethylene glycol loss, 32 Da from O2 loss, 138 Da from hydroxybenzoic acid, and 120 Da from oxomethylene cyclohexadienone) provided more specific information about structures of the identified derivatives of carminic acid.
Figures



Similar articles
-
A Mass Spectrometry-Based Approach for Characterization of Red, Blue, and Purple Natural Dyes.Molecules. 2020 Jul 15;25(14):3223. doi: 10.3390/molecules25143223. Molecules. 2020. PMID: 32679693 Free PMC article.
-
Investigation of red natural dyes used in historical objects by HPLC-DAD-MS.Ann Chim. 2006 Jan-Feb;96(1-2):75-84. doi: 10.1002/adic.200690008. Ann Chim. 2006. PMID: 16736555
-
Spiroketalcarminic Acid, a Novel Minor Anthraquinone Pigment in Cochineal Extract Used in Food Additives.Chem Pharm Bull (Tokyo). 2017 Sep 1;65(9):883-887. doi: 10.1248/cpb.c17-00404. Epub 2017 Jul 1. Chem Pharm Bull (Tokyo). 2017. PMID: 28674282
-
Analysis of natural red dyes (cochineal) in textiles of historical importance using HPLC and multivariate data analysis.Anal Bioanal Chem. 2011 Aug;401(2):735-43. doi: 10.1007/s00216-011-5094-0. Epub 2011 May 31. Anal Bioanal Chem. 2011. PMID: 21626194
-
The Use of Insect Pigment in Art Works.Insects. 2024 Jul 10;15(7):519. doi: 10.3390/insects15070519. Insects. 2024. PMID: 39057252 Free PMC article. Review.
Cited by
-
Heterologous production of the widely used natural food colorant carminic acid in Aspergillus nidulans.Sci Rep. 2018 Aug 27;8(1):12853. doi: 10.1038/s41598-018-30816-9. Sci Rep. 2018. PMID: 30150747 Free PMC article.
-
Mass Spectrometry for Investigation of Natural Dyes in Historical Textiles: Unveiling the Mystery behind Safflower-Dyed Fibers.J Am Soc Mass Spectrom. 2021 Oct 6;32(10):2552-2566. doi: 10.1021/jasms.1c00195. Epub 2021 Sep 3. J Am Soc Mass Spectrom. 2021. PMID: 34478285 Free PMC article.
-
Multi-Analytical Techniques for the Study of Burial Clothes of Polish King Sigismund III Vasa (1566-1633) and His Wife Constance Habsburg (1588-1631).Molecules. 2023 Dec 28;29(1):192. doi: 10.3390/molecules29010192. Molecules. 2023. PMID: 38202776 Free PMC article.
-
A Mass Spectrometry-Based Approach for Characterization of Red, Blue, and Purple Natural Dyes.Molecules. 2020 Jul 15;25(14):3223. doi: 10.3390/molecules25143223. Molecules. 2020. PMID: 32679693 Free PMC article.
-
Characterization of a membrane-bound C-glucosyltransferase responsible for carminic acid biosynthesis in Dactylopius coccus Costa.Nat Commun. 2017 Dec 7;8(1):1987. doi: 10.1038/s41467-017-02031-z. Nat Commun. 2017. PMID: 29215010 Free PMC article.
References
-
- Cardon D (2007) Natural dyes: sources, tradition, technology, science. Archetype, London
-
- Phipps E. Cochineal red: the art history of a color. New York: The Metropolitan Museum of Art; 2010.
-
- Hofenk de Graaff JH (2004) The colorful past. Origins, chemistry and identification of natural dyestuffs. Archetype, London
-
- Lech K, Połeć-Pawlak K, Jarosz M. Mass spectrometry in identification of color components of natural organic dyestuffs used in art. Chem Anal. 2008;53:479–509.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

