Sister kinetochores are mechanically fused during meiosis I in yeast
- PMID: 25213378
- PMCID: PMC4226495
- DOI: 10.1126/science.1256729
Sister kinetochores are mechanically fused during meiosis I in yeast
Abstract
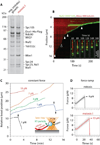
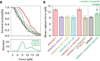
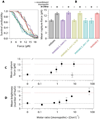
Production of healthy gametes requires a reductional meiosis I division in which replicated sister chromatids comigrate, rather than separate as in mitosis or meiosis II. Fusion of sister kinetochores during meiosis I may underlie sister chromatid comigration in diverse organisms, but direct evidence for such fusion has been lacking. We used laser trapping and quantitative fluorescence microscopy to study native kinetochore particles isolated from yeast. Meiosis I kinetochores formed stronger attachments and carried more microtubule-binding elements than kinetochores isolated from cells in mitosis or meiosis II. The meiosis I-specific monopolin complex was both necessary and sufficient to drive these modifications. Thus, kinetochore fusion directs sister chromatid comigration, a conserved feature of meiosis that is fundamental to Mendelian inheritance.
Copyright © 2014, American Association for the Advancement of Science.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
- P30 CA015704/CA/NCI NIH HHS/United States
- 096078/WT_/Wellcome Trust/United Kingdom
- R01GM104141/GM/NIGMS NIH HHS/United States
- 103139/Z/13/Z/WT_/Wellcome Trust/United Kingdom
- R01 GM104141/GM/NIGMS NIH HHS/United States
- R01GM064386/GM/NIGMS NIH HHS/United States
- R01 GM064386/GM/NIGMS NIH HHS/United States
- 103139/WT_/Wellcome Trust/United Kingdom
- 077707/WT_/Wellcome Trust/United Kingdom
- R01GM079373/GM/NIGMS NIH HHS/United States
- 090903/WT_/Wellcome Trust/United Kingdom
- R01 GM079373/GM/NIGMS NIH HHS/United States
- 084229/WT_/Wellcome Trust/United Kingdom
- 091020/WT_/Wellcome Trust/United Kingdom
- S10 RR026406/RR/NCRR NIH HHS/United States
- S10RR026406/RR/NCRR NIH HHS/United States
- 092076/WT_/Wellcome Trust/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

