LC-MS/MS Analysis of Cerebrospinal Fluid Metabolites in the Pterin Biosynthetic Pathway
- PMID: 25213568
- PMCID: PMC5059177
- DOI: 10.1007/8904_2014_336
LC-MS/MS Analysis of Cerebrospinal Fluid Metabolites in the Pterin Biosynthetic Pathway
Erratum in
-
Erratum to: LC-MS/MS Analysis of Cerebrospinal Fluid Metabolites in the Pterin Biosynthetic Pathway.JIMD Rep. 2016;29:115. doi: 10.1007/8904_2014_372. Epub 2016 Jun 8. JIMD Rep. 2016. PMID: 27272191 Free PMC article. No abstract available.
Abstract
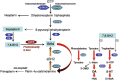
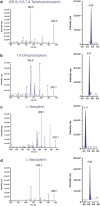
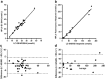
The analysis of (6R)-5,6,7,8-tetrahydrobiopterin (BH4) and neopterin in cerebrospinal fluid (CSF) is often used to identify defects in the pterin biosynthetic pathway affecting monoamine metabolism that can lead to pediatric neurotransmitter diseases. Low levels of BH4 and neopterin alone may not be sufficient to determine the defect, and further testing is often required. We have developed a sensitive liquid chromatography tandem mass spectrometry (LC-MS/MS) method for determination of BH4, 7,8-dihydrobiopterin (BH2), neopterin, and sepiapterin in CSF, which provides a more comprehensive evaluation of the pterin pathway. The method utilizes labeled stable isotopes as internal standards and allows for a fast 10-minute analysis by LC/MS/MS over a linear working range of 3 to 200 nmol/L. Total analytical imprecision is less than 14.4% for all pterin metabolites. Accuracy for BH4 and neopterin was determined by comparing data obtained by an alternative method using HPLC with EC and fluorescence detection. Excellent correlation was demonstrated for BH4 (r = 0.9646, 1/slope = 0.9397; n = 28; concentration range 3 to 63 nmol/L) and neopterin (r = 0.9919, 1/slope = 0.9539; n = 13; concentration range 5 to 240 nmol/L). CSF specimens from patients diagnosed with inborn errors of sepiapterin reductase (SR), 6-pyruvoyl-tetrahydropterin synthase (PTPS), dihydropteridine reductase (DHPR), and guanosine triphosphate cyclohydrolase (GTPCH) have been analyzed, and distinct pterin metabolite patterns were consistent with the initial diagnosis. This method differentiates patients with DHPR and SR deficiency from other pterin defects (GTPCH and PTPS) and will be useful for the diagnosis of specific defects in the pterin biosynthetic pathway.
Figures
Similar articles
-
Diagnosis of dopa-responsive dystonia and other tetrahydrobiopterin disorders by the study of biopterin metabolism in fibroblasts.Clin Chem. 2001 Mar;47(3):477-85. Clin Chem. 2001. PMID: 11238300
-
[Biopterin and child neurologic disease].No To Hattatsu. 2009 Jan;41(1):5-10. No To Hattatsu. 2009. PMID: 19172809 Review. Japanese.
-
Disorders of tetrahydrobiopterin metabolism and their treatment.Curr Drug Metab. 2002 Apr;3(2):123-31. doi: 10.2174/1389200024605145. Curr Drug Metab. 2002. PMID: 12003346 Review.
-
Analysis of Catecholamines and Pterins in Inborn Errors of Monoamine Neurotransmitter Metabolism-From Past to Future.Cells. 2019 Aug 9;8(8):867. doi: 10.3390/cells8080867. Cells. 2019. PMID: 31405045 Free PMC article. Review.
-
Tetrahydrobiopterin deficiencies: Lesson from clinical experience.JIMD Rep. 2021 Feb 1;59(1):42-51. doi: 10.1002/jmd2.12199. eCollection 2021 May. JIMD Rep. 2021. PMID: 33977029 Free PMC article.
Cited by
-
Single-Step Rapid Diagnosis of Dopamine and Serotonin Metabolism Disorders.ACS Omega. 2017 Sep 30;2(9):5962-5972. doi: 10.1021/acsomega.7b01008. Epub 2017 Sep 19. ACS Omega. 2017. PMID: 30023757 Free PMC article.
-
Intestinal microbiota as a tetrahydrobiopterin exogenous source in hph-1 mice.Sci Rep. 2017 Jan 12;7:39854. doi: 10.1038/srep39854. Sci Rep. 2017. PMID: 28079055 Free PMC article.
-
The Utility of CSF for the Diagnosis of Primary and Secondary Monoamine Neurotransmitter Deficiencies.EJIFCC. 2017 Mar 8;28(1):64-76. eCollection 2017 Mar. EJIFCC. 2017. PMID: 28439219 Free PMC article.
-
Measurement of Tetrahydrobiopterin in Animal Tissue Samples by HPLC with Electrochemical Detection-Protocol Optimization and Pitfalls.Antioxidants (Basel). 2022 Jun 16;11(6):1182. doi: 10.3390/antiox11061182. Antioxidants (Basel). 2022. PMID: 35740082 Free PMC article.
-
Elucidation of the complex metabolic profile of cerebrospinal fluid using an untargeted biochemical profiling assay.Mol Genet Metab. 2017 Jun;121(2):83-90. doi: 10.1016/j.ymgme.2017.04.005. Epub 2017 Apr 9. Mol Genet Metab. 2017. PMID: 28412083 Free PMC article.
References
-
- Blau N, Thöny B, Cotton RGH, Hyland K. Disorders of tetrahydrobiopterin and related biogenic amines. In: Scriver CR, Beaudet AL, Sly WS, Valle D, Vogelstein B, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001. pp. 1725–1776.
-
- Bräutigam M, Dreesen R, Herken H. Determination of reduced biopterins by high pressure liquid chromatography and subsequent electrochemical detection. Hoppe Seylers Z Physiol Chem. 1982;363(3):341–343. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

