Response to cholinesterase inhibitors affects lifespan in Alzheimer's disease
- PMID: 25213579
- PMCID: PMC4172846
- DOI: 10.1186/s12883-014-0173-4
Response to cholinesterase inhibitors affects lifespan in Alzheimer's disease
Abstract
Background: A varying response to cholinesterase inhibitor (ChEI) treatment has been reported among patients with Alzheimer's disease (AD). Whether the individual-specific response, specific ChEI agent or dose affects mortality is unclear. We aimed to examine the relationship between the 6-month response to ChEI and lifespan.
Methods: Six hundred and eighty-one deceased patients with a clinical AD diagnosis and a Mini-Mental State Examination (MMSE) score of 10-26 at the start of ChEI therapy (baseline) were included in a prospective, observational, multicentre study in clinical practice. At baseline and after 6 months of treatment, the participants were assessed using the MMSE, the Alzheimer's Disease Assessment Scale-cognitive subscale (ADAS-cog), the Clinician's Interview-Based Impression of Change (CIBIC), the Instrumental Activities of Daily Living (IADL) scale, and the Physical Self-Maintenance Scale (PSMS). The individuals' socio-demographic characteristics, ChEI dose, and date of death were recorded. Responses to ChEI and the association of possible risk factors with survival were analysed using general linear models.
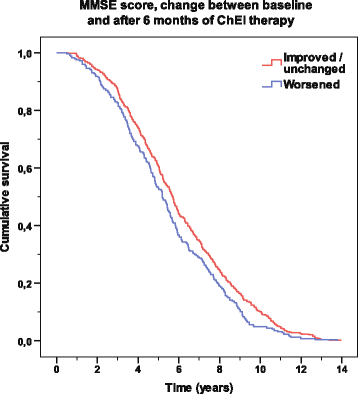
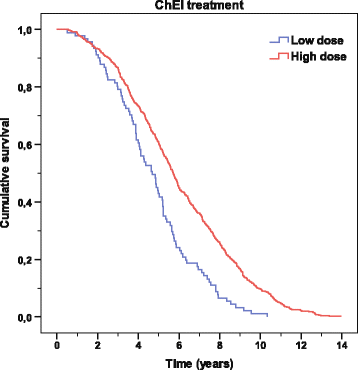
Results: A longer lifespan (mean of 0.5 years) was observed among the improved/unchanged patients, as measured by MMSE or CIBIC score, but not by ADAS-cog score, after 6 months of ChEI therapy. In the multivariate models, increased survival time was independently related to a better 6-month response in MMSE, CIBIC, IADL, and PSMS scores, female sex, no antihypertensive/cardiac or antidiabetic therapy, younger age, lower education, milder disease stage at baseline, and higher ChEI dose. Apolipoprotein E genotype did not affect mortality significantly. The patients who received a higher ChEI dose during the first 6 months had a mean lifespan after baseline that was 15 months longer than that of those who received a lower dose.
Conclusions: A better short-term response to ChEI might prolong survival in naturalistic AD patients. In individuals who received and tolerated higher ChEI doses, a longer lifespan can be expected.
Figures
Similar articles
-
Early- versus late-onset Alzheimer's disease in clinical practice: cognitive and global outcomes over 3 years.Alzheimers Res Ther. 2017 Aug 31;9(1):70. doi: 10.1186/s13195-017-0294-2. Alzheimers Res Ther. 2017. PMID: 28859660 Free PMC article.
-
Functional response to cholinesterase inhibitor therapy in a naturalistic Alzheimer's disease cohort.BMC Neurol. 2012 Nov 5;12:134. doi: 10.1186/1471-2377-12-134. BMC Neurol. 2012. PMID: 23126532 Free PMC article. Clinical Trial.
-
Mild versus moderate stages of Alzheimer's disease: three-year outcomes in a routine clinical setting of cholinesterase inhibitor therapy.Alzheimers Res Ther. 2016 Feb 17;8:7. doi: 10.1186/s13195-016-0174-1. Alzheimers Res Ther. 2016. PMID: 26883213 Free PMC article.
-
Pharmacotherapeutic approaches to the treatment of Alzheimer's disease.Clin Ther. 2004 May;26(5):615-30. doi: 10.1016/s0149-2918(04)90064-1. Clin Ther. 2004. PMID: 15220008 Review.
-
Prediction models for assessing long-term outcome in Alzheimer's disease: a review.Am J Alzheimers Dis Other Demen. 2013 Aug;28(5):440-9. doi: 10.1177/1533317513488916. Epub 2013 May 20. Am J Alzheimers Dis Other Demen. 2013. PMID: 23689074 Free PMC article. Review.
Cited by
-
Can Persistence With Cholinesterase Inhibitor Treatment Lower Mortality and Health-Care Costs Among Patients With Alzheimer's Disease? A Population-Based Study in Taiwan.Am J Alzheimers Dis Other Demen. 2018 Mar;33(2):86-92. doi: 10.1177/1533317517734639. Epub 2017 Dec 6. Am J Alzheimers Dis Other Demen. 2018. PMID: 29210284 Free PMC article.
-
Long-term Effects of Cholinesterase Inhibitors on Cognitive Decline and Mortality.Neurology. 2021 Apr 27;96(17):e2220-e2230. doi: 10.1212/WNL.0000000000011832. Epub 2021 Mar 19. Neurology. 2021. PMID: 33741639 Free PMC article.
-
Gender Differences in Demographic and Pharmacological Factors in Patients Diagnosed with Late-Onset of Alzheimer's Disease.Brain Sci. 2022 Jan 26;12(2):160. doi: 10.3390/brainsci12020160. Brain Sci. 2022. PMID: 35203924 Free PMC article.
-
Toxicological Differences Between NMDA Receptor Antagonists and Cholinesterase Inhibitors.Am J Alzheimers Dis Other Demen. 2016 Aug;31(5):405-12. doi: 10.1177/1533317515622283. Epub 2016 Jan 14. Am J Alzheimers Dis Other Demen. 2016. PMID: 26769920 Free PMC article.
-
Combined use of Donepezil and Memantine increases the probability of five-year survival of Alzheimer's disease patients.Commun Med (Lond). 2024 May 23;4(1):99. doi: 10.1038/s43856-024-00527-6. Commun Med (Lond). 2024. PMID: 38783011 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

