A conformational sampling model for radical catalysis in pyridoxal phosphate- and cobalamin-dependent enzymes
- PMID: 25213862
- PMCID: PMC4256349
- DOI: 10.1074/jbc.M114.590471
A conformational sampling model for radical catalysis in pyridoxal phosphate- and cobalamin-dependent enzymes
Abstract
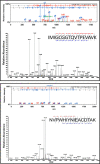
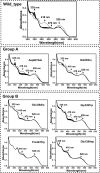
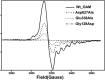
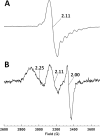
Cobalamin-dependent enzymes enhance the rate of C-Co bond cleavage by up to ∼10(12)-fold to generate cob(II)alamin and a transient adenosyl radical. In the case of the pyridoxal 5'-phosphate (PLP) and cobalamin-dependent enzymes lysine 5,6-aminomutase and ornithine 4,5 aminomutase (OAM), it has been proposed that a large scale domain reorientation of the cobalamin-binding domain is linked to radical catalysis. Here, OAM variants were designed to perturb the interface between the cobalamin-binding domain and the PLP-binding TIM barrel domain. Steady-state and single turnover kinetic studies of these variants, combined with pulsed electron-electron double resonance measurements of spin-labeled OAM were used to provide direct evidence for a dynamic interface between the cobalamin and PLP-binding domains. Our data suggest that following ligand binding-induced cleavage of the Lys(629)-PLP covalent bond, dynamic motion of the cobalamin-binding domain leads to conformational sampling of the available space. This supports radical catalysis through transient formation of a catalytically competent active state. Crucially, it appears that the formation of the state containing both a substrate/product radical and Co(II) does not restrict cobalamin domain motion. A similar conformational sampling mechanism has been proposed to support rapid electron transfer in a number of dynamic redox systems.
Keywords: Adenosylcobalamin (AdoCbl); Conformational Sampling; Domain Dynamics; Electron Paramagnetic Resonance (EPR); Ornithine Aminomutase; Protein Dynamic; Pyridoxal Phosphate; Radical.
© 2014 by The American Society for Biochemistry and Molecular Biology, Inc.
Figures







References
-
- Brown K. L. (2005) Chemistry and enzymology of vitamin B12. Chem. Rev. 105, 2075–2149 - PubMed
-
- Marsh E. N. (1995) A radical approach to enzyme catalysis. Bioessays 17, 431–441 - PubMed
-
- Roth J. R., Lawrence J. G., Bobik T. A. (1996) Cobalamin (coenzyme B12): synthesis and biological significance. Annu. Rev. Microbiol. 50, 137–181 - PubMed
-
- Stubbe J. A. (1989) Protein radical involvement in biological catalysis? Annu. Rev. Biochem. 58, 257–285 - PubMed
-
- Marsh E. N., Ballou D. P. (1998) Coupling of cobalt-carbon bond homolysis and hydrogen atom abstraction in adenosylcobalamin-dependent glutamate mutase. Biochemistry 37, 11864–11872 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

