Presence of intramucosal neuroglial cells in normal and aganglionic human colon
- PMID: 25214400
- PMCID: PMC7864228
- DOI: 10.1152/ajpgi.00164.2014
Presence of intramucosal neuroglial cells in normal and aganglionic human colon
Abstract
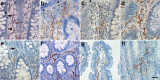
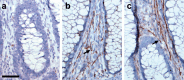
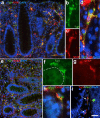
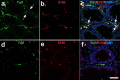
The enteric nervous system (ENS) is composed of neural crest-derived neurons (also known as ganglion cells) the cell bodies of which are located in the submucosal and myenteric plexuses of the intestinal wall. Intramucosal ganglion cells are known to exist but are rare and often considered ectopic. Also derived from the neural crest are enteric glial cells that populate the ganglia and the associated nerves, as well as the lamina propria of the intestinal mucosa. In Hirschsprung disease (HSCR), ganglion cells are absent from the distal gut because of a failure of neural crest-derived progenitor cells to complete their rostrocaudal migration during embryogenesis. The fate of intramucosal glial cells in human HSCR is essentially unknown. We demonstrate a network of intramucosal cells that exhibit dendritic morphology typical of neurons and glial cells. These dendritic cells are present throughout the human gut and express Tuj1, S100, glial fibrillary acidic protein, CD56, synaptophysin, and calretinin, consistent with mixed or overlapping neuroglial differentiation. The cells are present in aganglionic colon from patients with HSCR, but with an altered immunophenotype. Coexpression of Tuj1 and HNK1 in this cell population supports a neural crest origin. These findings extend and challenge the current understanding of ENS microanatomy and suggest the existence of an intramucosal population of neural crest-derived cells, present in HSCR, with overlapping immunophenotype of neurons and glia. Intramucosal neuroglial cells have not been previously recognized, and their presence in HSCR poses new questions about ENS development and the pathobiology of HSCR that merit further investigation.
Keywords: Hirschsprung disease; enteric glial cells; enteric nervous system.
Copyright © 2014 the American Physiological Society.
Figures




References
-
- Akbar A, Yiangou Y, Facer P, Brydon WG, Walters JR, Anand P, Ghosh S. Expression of the TRPV1 receptor differs in quiescent inflammatory bowel disease with or without abdominal pain. Gut : 767–774, 2010. - PubMed
-
- Balemba OB, Grondahl ML, Mbassa GK, Semuguruka WD, Hay-Smith A, Skadhauge E, Dantzer V. The organisation of the enteric nervous system in the submucous and mucous layers of the small intestine of the pig studied by VIP and neurofilament protein immunohistochemistry. J Anat : 257–267, 1998. - PMC - PubMed
-
- Burns AJ, Douarin NM. The sacral neural crest contributes neurons and glia to the post-umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development : 4335–4347, 1998. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

