Prolonged exposure of cholestatic rats to complete dark inhibits biliary hyperplasia and liver fibrosis
- PMID: 25214401
- PMCID: PMC4216989
- DOI: 10.1152/ajpgi.00288.2014
Prolonged exposure of cholestatic rats to complete dark inhibits biliary hyperplasia and liver fibrosis
Abstract
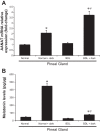
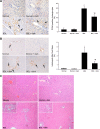
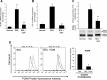
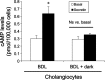
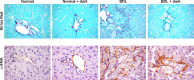
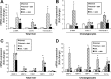
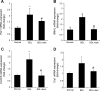
Biliary hyperplasia and liver fibrosis are common features in cholestatic liver disease. Melatonin is synthesized by the pineal gland as well as the liver. Melatonin inhibits biliary hyperplasia of bile duct-ligated (BDL) rats. Since melatonin synthesis (by the enzyme serotonin N-acetyltransferase, AANAT) from the pineal gland increases after dark exposure, we hypothesized that biliary hyperplasia and liver fibrosis are diminished by continuous darkness via increased melatonin synthesis from the pineal gland. Normal or BDL rats (immediately after surgery) were housed with light-dark cycles or complete dark for 1 wk before evaluation of 1) the expression of AANAT in the pineal gland and melatonin levels in pineal gland tissue supernatants and serum; 2) biliary proliferation and intrahepatic bile duct mass, liver histology, and serum chemistry; 3) secretin-stimulated ductal secretion (functional index of biliary growth); 4) collagen deposition, liver fibrosis markers in liver sections, total liver, and cholangiocytes; and 5) expression of clock genes in cholangiocytes. In BDL rats exposed to dark there was 1) enhanced AANAT expression/melatonin secretion in pineal gland and melatonin serum levels; 2) improved liver morphology, serum chemistry and decreased biliary proliferation and secretin-stimulated choleresis; and 4) decreased fibrosis and expression of fibrosis markers in liver sections, total liver and cholangiocytes and reduced biliary expression of the clock genes PER1, BMAL1, CLOCK, and Cry1. Thus prolonged dark exposure may be a beneficial noninvasive therapeutic approach for the management of biliary disorders.
Keywords: biliary epithelium; cholestasis; clock genes; melatonin; secretin.
Figures
References
-
- Alpini G, Glaser S, Robertson W, Rodgers RE, Phinizy JL, Lasater J, LeSage GD. Large but not small intrahepatic bile ducts are involved in secretin-regulated ductal bile secretion. Am J Physiol Gastrointest Liver Physiol 272: G1064–G1074, 1997. - PubMed
-
- Alpini G, Glaser S, Ueno Y, Pham L, Podila PV, Caligiuri A, LeSage G, LaRusso NF. Heterogeneity of the proliferative capacity of rat cholangiocytes after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 274: G767–G775, 1998. - PubMed
-
- Alpini G, Roberts S, Kuntz SM, Ueno Y, Gubba S, Podila PV, LeSage G, LaRusso NF. Morphological, molecular, and functional heterogeneity of cholangiocytes from normal rat liver. Gastroenterology 110: 1636–1643, 1996. - PubMed
-
- Alpini G, Ulrich C, Roberts S, Phillips JO, Ueno Y, Podila PV, Colegio O, LeSage G, Miller LJ, LaRusso NF. Molecular and functional heterogeneity of cholangiocytes from rat liver after bile duct ligation. Am J Physiol Gastrointest Liver Physiol 272: G289–G297, 1997. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

