Intracellular ATP does not inhibit Slo2.1 K+ channels
- PMID: 25214519
- PMCID: PMC4270230
- DOI: 10.14814/phy2.12118
Intracellular ATP does not inhibit Slo2.1 K+ channels
Abstract
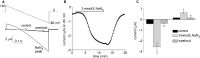
Under normal physiological conditions, the open probability of Slo2.1 K(+) channels is low. Elevation of cytosolic [Na(+)] and [Cl(-)] caused by ischemia or rapid electrical pacing of cells increases the open probability of Slo2.1 channels and the resulting outward current can stabilize the resting state of cells. Initial characterization of heterologously expressed human Slo2.1 indicated that these channels were inhibited by physiological levels of intracellular ATP. However, a subsequent study found that intracellular ATP had no effect on Slo2.1 channels. Here, we re-examine the effects of intracellular ATP on cloned human Slo2.1 channels heterologously expressed in Xenopus oocytes. Our studies provide both direct and indirect evidence that changes in intracellular [ATP] have no effect on Slo2.1 channels. First, we directly examined the effects of intracellular ATP on Slo2.1 channel activity in excised inside-out macropatches from Xenopus oocytes. Application of 5 mmol/L ATP to the intracellular solution did not inhibit Slo2.1 currents activated by niflumic acid. Second, we lowered the [ATP]i in whole oocytes using the metabolic inhibitor NaN3. Depletion of [ATP]i in oocytes by 3 mmol/L NaN3 rapidly activated heterologously expressed KATP channels, but did not increase wild-type Slo2.1 channel currents activated by niflumic acid or currents conducted by constitutively active mutant (E275D) Slo2.1 channels. Third, mutation of a conserved residue in the ATP binding consensus site in the C-terminal domain of the channel did not enhance the magnitude of Slo2.1 current as expected if binding to this site inhibited channel function. We conclude that Slo2.1 channels are not inhibited by intracellular ATP.
Keywords: ATP; KCNT2; Xenopus; oocytes; potassium channels.
© 2014 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures



Similar articles
-
Identification of the Intracellular Na+ Sensor in Slo2.1 Potassium Channels.J Biol Chem. 2015 Jun 5;290(23):14528-35. doi: 10.1074/jbc.M115.653089. Epub 2015 Apr 22. J Biol Chem. 2015. PMID: 25903137 Free PMC article.
-
Activation of Slo2.1 channels by niflumic acid.J Gen Physiol. 2010 Mar;135(3):275-95. doi: 10.1085/jgp.200910316. J Gen Physiol. 2010. PMID: 20176855 Free PMC article.
-
Hydrophobic interactions between the S5 segment and the pore helix stabilizes the closed state of Slo2.1 potassium channels.Biochim Biophys Acta. 2016 Apr;1858(4):783-92. doi: 10.1016/j.bbamem.2015.12.024. Epub 2015 Dec 23. Biochim Biophys Acta. 2016. PMID: 26724206 Free PMC article.
-
ATP sensitive potassium channel and myocardial preconditioning.Acta Anaesthesiol Sin. 1999 Sep;37(3):121-31. Acta Anaesthesiol Sin. 1999. PMID: 10609345 Review.
-
Mechanisms of cellular synchronization in the vascular wall. Mechanisms of vasomotion.Dan Med Bull. 2010 Oct;57(10):B4191. Dan Med Bull. 2010. PMID: 21040688 Review.
Cited by
-
The Epilepsy of Infancy With Migrating Focal Seizures: Identification of de novo Mutations of the KCNT2 Gene That Exert Inhibitory Effects on the Corresponding Heteromeric KNa1.1/KNa1.2 Potassium Channel.Front Cell Neurosci. 2020 Jan 24;14:1. doi: 10.3389/fncel.2020.00001. eCollection 2020. Front Cell Neurosci. 2020. PMID: 32038177 Free PMC article.
-
The Slo(w) path to identifying the mitochondrial channels responsible for ischemic protection.Biochem J. 2017 Jun 9;474(12):2067-2094. doi: 10.1042/BCJ20160623. Biochem J. 2017. PMID: 28600454 Free PMC article. Review.
-
Structural basis and synergism of ATP and Na+ activation in bacterial K+ uptake system KtrAB.Nat Commun. 2024 May 8;15(1):3850. doi: 10.1038/s41467-024-48057-y. Nat Commun. 2024. PMID: 38719864 Free PMC article.
-
International Union of Basic and Clinical Pharmacology. C. Nomenclature and Properties of Calcium-Activated and Sodium-Activated Potassium Channels.Pharmacol Rev. 2017 Jan;69(1):1-11. doi: 10.1124/pr.116.012864. Epub 2016 Nov 15. Pharmacol Rev. 2017. PMID: 28267675 Free PMC article. Review.
-
Identification of the Intracellular Na+ Sensor in Slo2.1 Potassium Channels.J Biol Chem. 2015 Jun 5;290(23):14528-35. doi: 10.1074/jbc.M115.653089. Epub 2015 Apr 22. J Biol Chem. 2015. PMID: 25903137 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

