Mechanistic Evaluation of the Ni(IPr)2-Catalyzed Cycloaddition of Alkynes and Nitriles to Afford Pyridines: Evidence for the Formation of a Key η1-Ni(IPr)2(RCN) Intermediate
- PMID: 25214702
- PMCID: PMC4159214
- DOI: 10.1021/om400666k
Mechanistic Evaluation of the Ni(IPr)2-Catalyzed Cycloaddition of Alkynes and Nitriles to Afford Pyridines: Evidence for the Formation of a Key η1-Ni(IPr)2(RCN) Intermediate
Abstract
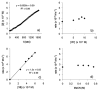
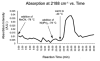
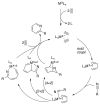
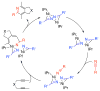
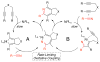
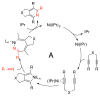
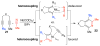
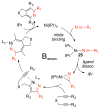
A detailed mechanistic evaluation of the Ni(IPr)2-catalyzed [2+2+2]-cycloaddition of diynes and nitriles was 2 conducted. Through kinetic analysis of these reactions, observed regioselectivities of the products, and stoichiometric reactions, Ni(IPr)2-catalyzed cycloadditions of diynes and nitriles appear to proceed by a heterooxidative coupling mechanism, contrary to other common cycloaddition catalysts. Reaction profiles demonstrated strong dependence in nitrile, resulting in variable nitrile-dependent resting states. Strong coordination and considerable steric bulk of the carbene ligands facilitate selective initial binding of nitrile thereby forcing a heterocoupling pathway. In situ IR data suggests the initial binding of the nitrile resides in a rare, η1-bound conformation. Following nitrile coordination are a rate-determining hapticity shift of the nitrile and subsequent loss of carbene. Alkyne coordination then leads to heterooxidative coupling, insertion of the pendant alkyne, and reductive elimination to afford pyridine products.
Figures



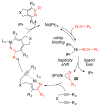

References
-
-
For reviews see: Varela JA, Saá C. Synlett. 2008;17:2571.. Heller B, Hapke M. Chem Soc Rev. 2007;36:1085.. Henry GD. Tetrahedron. 2004;60:6043.. Varela JA, Saá C. Chem Rev. 2003;103:3787.
-
-
-
For leading sources see: Jones G. Pyridines and Their Benzoderivatives: Synthesis. In: Katritzky A, Rees CW, Scriven EFV, editors. Comprehensive Heterocyclic Chemistry II. Vol. 5. Pergamo; Oxford: 1996. p. 167.. Alford PE. SixMembered Ring Systems: Pyridines and Their Benzo Derivatives. In: Gribble GW, Joule JA, editors. Progress in Heterocyclic Chemistry. Vol. 22. Elsevier; Oxford: 2011. p. 349.. Gonzalez-Bello C, Castedo L. SixMembered Heterocycles: Pyridines. In: Alvarez-Builla J, Vaquero JJ, Barluenga J, editors. Modern Heterocyclic Chemistry. Vol. 3. Wiley-VHC; Weinheim: 2011. p. 1431.
-
-
-
For general review of transition metal–catalyzed [2+2+2]-cycloaddition application in natural products for both carbo- and heterocyclic products see: Kotha S, Brahmachary E, Lahiri K. Eur J Org Chem. 2005:4741.. For selected applications in heterocycle synthesis see: Earl RA, Vollhardt KPC. J Org Chem. 1984;49:4786.. Parnell CA, Vollhardt KPC. Tetrahedron. 1985;41:5791.. Saá C, Crotts DD, Hsu G, Vollhardt KPC. Synlett. 1994:487.. Andrew LM, Deiters A. Org Lett. 2010;12:1288–1291.. Alayrac C, Schollmeyer D, Witulski B. Chem Commun. 2009:1464–1466..
-
-
-
For leading sources for Co-catalyzed systems see: Vollhardt KPC. Angew Chem. 1984;96:525.. Bönnemann H. Angew Chem Int Ed Engl. 1985;24:248.. Bönnemann H, Brijoux W. Adv Heterocycl Chem. 1990;48:177.. Garcia P, Evanno Y, George P, Sevrin M, Ricci M, Malacria M, Aubert C, Gandon V. Chem Eur J. 2012;18:4337.. For Ru-systems: Yamamoto Y, Ogawa R, Itoh K. J Am Chem Soc. 2001;123:6189.. Varela JA, Castedo L, Saa C. J Org Chem. 2003;68:8595.. Yamamoto Y, Kinpara K, Saigoku T, Takagishi H, Okuda S, Nishiyama H, Itoh K. J Am Chem Soc. 2005;127:605.. Calhorda MJ, Costa PJ, Kirchner KA. Inorg Chem Acta. 2011;374:24.. For Ir-system: Onodera G, Shimizu Y, Kimura JN, Kobayashi J, Ebihara Y, Kondo K, Sakata K, Takeuchi R. J Am Chem Soc. 2012;134:10515.
-
-
- McCormick MM, Duong HA, Zuo G, Louie J. J Am Chem Soc. 2005;127:5030. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
