The study of mechanisms of protective effect of Rg1 against arthritis by inhibiting osteoclast differentiation and maturation in CIA mice
- PMID: 25214714
- PMCID: PMC4158307
- DOI: 10.1155/2014/305071
The study of mechanisms of protective effect of Rg1 against arthritis by inhibiting osteoclast differentiation and maturation in CIA mice
Abstract
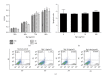
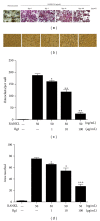
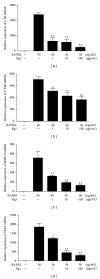
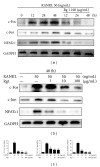
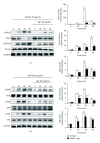
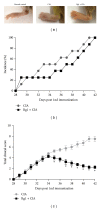
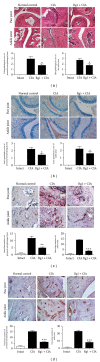
Ginsenoside Rg1 is a natural product extracted from Panax ginseng C.A. Although Rg1 protects tissue structure and functions by inhibiting local inflammatory reaction, the mechanism remains poorly understood. In vitro, Rg1 dose-dependently inhibited TRAP activity in receptor activator of nuclear factor-κB ligand- (RANKL-) induced osteoclasts and decreased the number of osteoclasts and osteoclast resorption area. Rg1 also significantly inhibited the RANK signaling pathway, including suppressing the expression of Trap, cathepsin K, matrix metalloproteinase 9 (MMP9), and calcitonin receptor (CTR). In vivo, Rg1 dramatically decreased arthritis scores in CIA mice and effectively controlled symptoms of inflammatory arthritis. Pathologic analysis demonstrated that Rg1 significantly attenuated pathological changes in CIA mice. Pronounced reduction in synovial hyperplasia and inflammatory cell invasion were observed in CIA mice after Rg1 therapy. Alcian blue staining results illustrated that mice treated with Rg1 had significantly reduced destruction in the articular cartilage. TRAP and cathepsin K staining results demonstrated a significant reduction of numbers of OCs in the articular cartilage in proximal interphalangeal joints and ankle joints in Rg1-treated mice. In summary, this study revealed that Rg1 reduced the inflammatory destruction of periarticular bone by inhibiting differentiation and maturation of osteoclasts in CIA mice.
Figures
Similar articles
-
Boldine isolated from Litsea cubeba inhibits bone resorption by suppressing the osteoclast differentiation in collagen-induced arthritis.Int Immunopharmacol. 2017 Oct;51:114-123. doi: 10.1016/j.intimp.2017.08.013. Epub 2017 Aug 18. Int Immunopharmacol. 2017. PMID: 28826044
-
Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis.Am J Pathol. 2002 Oct;161(4):1419-27. doi: 10.1016/S0002-9440(10)64417-3. Am J Pathol. 2002. PMID: 12368214 Free PMC article.
-
Protection against cartilage and bone destruction by systemic interleukin-4 treatment in established murine type II collagen-induced arthritis.Arthritis Res. 1999;1(1):81-91. doi: 10.1186/ar14. Epub 1999 Oct 26. Arthritis Res. 1999. PMID: 11056663 Free PMC article.
-
Celastrol attenuates bone erosion in collagen-Induced arthritis mice and inhibits osteoclast differentiation and function in RANKL-induced RAW264.7.Int Immunopharmacol. 2015 Feb;24(2):239-246. doi: 10.1016/j.intimp.2014.12.012. Epub 2014 Dec 19. Int Immunopharmacol. 2015. PMID: 25529994
-
Prolactin blocks the expression of receptor activator of nuclear factor κB ligand and reduces osteoclastogenesis and bone loss in murine inflammatory arthritis.Arthritis Res Ther. 2017 May 15;19(1):93. doi: 10.1186/s13075-017-1290-4. Arthritis Res Ther. 2017. PMID: 28506283 Free PMC article.
Cited by
-
Intestinal microbiome-rheumatoid arthritis crosstalk: The therapeutic role of probiotics.Front Microbiol. 2022 Oct 18;13:996031. doi: 10.3389/fmicb.2022.996031. eCollection 2022. Front Microbiol. 2022. PMID: 36329845 Free PMC article. Review.
-
Bone-targeted methotrexate-alendronate conjugate inhibits osteoclastogenesis in vitro and prevents bone loss and inflammation of collagen-induced arthritis in vivo.Drug Deliv. 2018 Nov;25(1):187-197. doi: 10.1080/10717544.2017.1422295. Drug Deliv. 2018. PMID: 29303005 Free PMC article.
-
Triptolide Inhibits Osteoclast Differentiation and Bone Resorption In Vitro via Enhancing the Production of IL-10 and TGF-β1 by Regulatory T Cells.Mediators Inflamm. 2016;2016:8048170. doi: 10.1155/2016/8048170. Epub 2016 Jun 19. Mediators Inflamm. 2016. PMID: 27413257 Free PMC article.
-
Lycopus lucidus Turcz Inhibits the Osteoclastogenesis in RAW 264.7 Cells and Bone Loss in Ovariectomized Rat Model.Evid Based Complement Alternat Med. 2019 Feb 21;2019:3231784. doi: 10.1155/2019/3231784. eCollection 2019. Evid Based Complement Alternat Med. 2019. PMID: 30915145 Free PMC article.
-
Bioactive strontium ions/ginsenoside Rg1-incorporated biodegradable silk fibroin-gelatin scaffold promoted challenging osteoporotic bone regeneration.Mater Today Bio. 2021 Sep 28;12:100141. doi: 10.1016/j.mtbio.2021.100141. eCollection 2021 Sep. Mater Today Bio. 2021. PMID: 34632364 Free PMC article.
References
-
- Ngian GS. Rheumatoid arthritis. Australian Family Physician. 2010;39(9):626–628. - PubMed
-
- Suda T, Takahashi N, Udagawa N, Jimi E, Gillespie MT, Martin TJ. Modulation of osteoclast differentiation and function by the new members of the tumor necrosis factor receptor and ligand families. Endocrine Reviews. 1999;20(3):345–357. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

