Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis
- PMID: 25214721
- PMCID: PMC4158168
- DOI: 10.1155/2014/973986
Metformin attenuates experimental autoimmune arthritis through reciprocal regulation of Th17/Treg balance and osteoclastogenesis
Abstract
Metformin is widely used to suppress certain functions of the cells found in diseases including diabetes and obesity. In this study, the effects of metformin on downregulating IL-17-producing T (Th17) cells, activating and upregulating regulatory T (Treg) cells, suppressing osteoclastogenesis, and clinically scoring collagen-induced arthritis (CIA) were investigated. To evaluate the effect of metformin on CIA, mice were orally fed with either metformin or saline as control three times a week for nine weeks. Histological analysis of the joints was performed using immunohistochemistry and Th17 cells and Treg cells of the spleen tissue were examined by confocal microscopy staining. Metformin mitigated the severity of CIA, reduced serum immunoglobulin concentrations, and reciprocally regulated Th17/Treg axis. Also, metformin treatment of normal cells cultured in Th17 conditions decreased the number of Th17 cells and increased the number of Treg cells. Metformin decreased gene expression and osteoclastogenic activity in CIA and normal mice. These results indicate that metformin had immunomodulatory actions influencing anti-inflammatory action on CIA through the inhibition of Th17 cell differentiation and the upregulation of Treg cell differentiation along with the suppression of osteoclast differentiation. Our results suggest that metformin may be a potential therapeutic for rheumatoid arthritis.
Figures
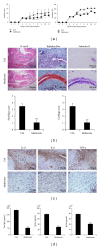





References
-
- Feldmann M, Brennan FM, Maini RN. Rheumatoid arthritis. Cell. 1996;85(3):307–310. - PubMed
-
- McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. The New England Journal of Medicine. 2011;365(23):2205–2219. - PubMed
-
- Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Current Opinion in Immunology. 2009;21(3):274–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

