The role of everolimus in liver transplantation
- PMID: 25214801
- PMCID: PMC4159129
- DOI: 10.2147/CEG.S41780
The role of everolimus in liver transplantation
Abstract
During the last 5 decades, liver transplantation has witnessed rapid development in terms of both technical and pharmacologic advances. Since their discovery, calcineurin inhibitors (CNIs) have remained the standard of care for immunosuppression therapy in liver transplantation, improving both patient and graft survival. However, adverse events, particularly posttransplant nephrotoxicity, associated with long-term CNI use have necessitated the development of alternate treatment approaches. These include combination therapy with a CNI and the inosine monophosphate dehydrogenase inhibitor mycophenolic acid and use of mammalian target of rapamycin (mTOR) inhibitors. Everolimus, a 40-O-(2-hydroxyethyl) derivative of mTOR inhibitor sirolimus, has a distinct pharmacokinetic profile. Several studies have assessed the role of everolimus in liver transplant recipients in combination with CNI reduction or as a CNI withdrawal strategy. The efficacy of everolimus-based immunosuppressive therapy has been demonstrated in both de novo and maintenance liver transplant recipients. A pivotal study in 719 de novo liver transplant recipients formed the basis of the recent approval of everolimus in combination with steroids and reduced-dose tacrolimus in liver transplantation. In this study, everolimus introduced at 30 days posttransplantation in combination with reduced-dose tacrolimus (exposure reduced by 39%) showed comparable efficacy (composite efficacy failure rate of treated biopsy-proven acute rejection, graft loss, or death) and achieved superior renal function as early as month 1 and maintained it over 2 years versus standard exposure tacrolimus. This review provides an overview of the efficacy and safety of everolimus-based regimens in liver transplantation in the de novo and maintenance settings, as well as in special populations such as patients with hepatocellular carcinoma recurrence, hepatitis C virus-positive patients, and pediatric transplant recipients. We also provide an overview of ongoing studies and discuss potential expansion of the role for everolimus in these settings.
Keywords: efficacy; everolimus; liver transplantation; mTOR inhibitors; safety.
Figures

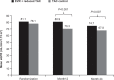

References
-
- Zarrinpar A, Busuttil RW. Liver transplantation: past, present and future. Nat Rev Gastroenterol Hepatol. 2013;10(7):434–440. - PubMed
-
- Wiesner R, Edwards E, Freeman R, et al. Model for end-stage liver disease (MELD) and allocation of donor livers. Gastroenterology. 2003;124(1):91–96. - PubMed
-
- Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med. 1996;334(11):693–699. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

