Kinetic Modeling Application to (18)F-fluoroethylcholine Positron Emission Tomography in Patients with Primary and Recurrent Prostate Cancer Using Two-tissue Compartmental Model
- PMID: 25214813
- PMCID: PMC4145150
- DOI: 10.4103/1450-1147.136734
Kinetic Modeling Application to (18)F-fluoroethylcholine Positron Emission Tomography in Patients with Primary and Recurrent Prostate Cancer Using Two-tissue Compartmental Model
Abstract
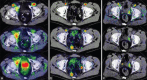
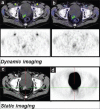
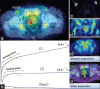
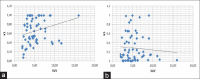
Although (18)F-fludeoxyglucose-positron emission tomography (PET) is the most applied diagnostic method in tumor staging, its role in prostate cancer (PCA) is limited because glucose metabolism tends to be low unless PCA has high Gleason score. Alternatively, choline PET was introduced as a valuable imaging method. Kinetic analysis of PET acquisition has increasingly gained momentum as an investigative tool because it provides a non-invasive approach to obtain kinetic and metabolic data from tissues of interest including transport and metabolism of the administered material. In this regard, we sought to apply it in (18)F-fluoroethylcholine (FECH)-PET/computed tomography (CT) in patients with PCA. 64 patients, the mean age 69 (range: 47-87 years) with primary/recurrent PCA were encompassed. They underwent (18)F-FECH-PET started with a dynamic acquisition using a 20-frame each 30 s over the prostate region and followed at 1 h post-injection by a static whole body imaging. The kinetic evaluation of the data was performed using the software package PMOD (PMOD Technologies Ltd., Zürich, Switzerland). Significant increase in mean values for K1, K3, FD, standardized uptake value (SUV) and global influx in tumor tissue versus normal tissue (P < 0.05). Moderate but significant correlation (r: 0.28, P = 0.023) between SUV and K1. By contrast, no correlation between SUV and K3 (r: -0.08, P = 0.79). In patients with recurrent tumors, there is no significant difference in all kinetic parameters and SUV (P > 0.1) between the different types of recurrences. The kinetic analysis of dynamic FECH-PET provides a novel method in primary PCA diagnosis and could be of potential value in the delineation of tumor focus.
Keywords: Choline-positron emission tomography; kinetic modeling; prostate cancer.
Conflict of interest statement
Figures



Similar articles
-
More advantages in detecting bone and soft tissue metastases from prostate cancer using 18F-PSMA PET/CT.Hell J Nucl Med. 2019 Jan-Apr;22(1):6-9. doi: 10.1967/s002449910952. Epub 2019 Mar 7. Hell J Nucl Med. 2019. PMID: 30843003
-
Quantitative 18F-fluorocholine positron emission tomography for prostate cancer: correlation between kinetic parameters and Gleason scoring.EJNMMI Res. 2017 Dec;7(1):25. doi: 10.1186/s13550-017-0269-0. Epub 2017 Mar 21. EJNMMI Res. 2017. PMID: 28324340 Free PMC article.
-
Quantitative studies using positron emission tomography (PET) for the diagnosis and therapy planning of oncological patients.Hell J Nucl Med. 2006 Jan-Apr;9(1):10-21. Hell J Nucl Med. 2006. PMID: 16617388
-
[18F]Fluoroethylcholine.2011 Mar 1 [updated 2011 Jun 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. 2011 Mar 1 [updated 2011 Jun 23]. In: Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US); 2004–2013. PMID: 21735583 Free Books & Documents. Review.
-
Imaging of prostate cancer with PET/CT using (18)F-Fluorocholine.Am J Nucl Med Mol Imaging. 2015 Jan 15;5(2):96-108. eCollection 2015. Am J Nucl Med Mol Imaging. 2015. PMID: 25973332 Free PMC article. Review.
Cited by
-
[18F]Fluorocholine PET/CT Imaging of Liver Cancer: Radiopathologic Correlation with Tissue Phospholipid Profiling.Mol Imaging Biol. 2017 Jun;19(3):446-455. doi: 10.1007/s11307-016-1020-3. Mol Imaging Biol. 2017. PMID: 27787742 Free PMC article. Clinical Trial.
-
Optimization of temporal sampling for 18F-choline uptake quantification in prostate cancer assessment.EJNMMI Res. 2018 Jun 15;8(1):49. doi: 10.1186/s13550-018-0410-8. EJNMMI Res. 2018. PMID: 29904817 Free PMC article.
-
18F Fluorocholine Dynamic Time-of-Flight PET/MR Imaging in Patients with Newly Diagnosed Intermediate- to High-Risk Prostate Cancer: Initial Clinical-Pathologic Comparisons.Radiology. 2017 Feb;282(2):429-436. doi: 10.1148/radiol.2016160220. Epub 2016 Aug 11. Radiology. 2017. PMID: 27513849 Free PMC article. Clinical Trial.
-
11C-Choline Pharmacokinetics in Recurrent Prostate Cancer.J Nucl Med. 2018 Nov;59(11):1672-1678. doi: 10.2967/jnumed.118.210088. Epub 2018 Apr 6. J Nucl Med. 2018. PMID: 29626123 Free PMC article.
-
Dynamic 11C-Choline PET / CT for the primary diagnosis of prostate cancer.Int Braz J Urol. 2018 Sep-Oct;44(5):900-905. doi: 10.1590/S1677-5538.IBJU.2018.0035. Int Braz J Urol. 2018. PMID: 30088719 Free PMC article.
References
-
- Oyama N, Akino H, Suzuki Y, Kanamaru H, Sadato N, Yonekura Y, et al. The increased accumulation of [18 F] fluorodeoxyglucose in untreated prostate cancer. Jpn J Clin Oncol. 1999;29:623–9. - PubMed
-
- Kanamaru H, Oyama N, Akino H, Okada K. Evaluation of prostate cancer using FDG-PET. Hinyokika Kiyo. 2000;46:851–3. - PubMed
-
- Beheshti M, Langsteger W, Fogelman I. Prostate cancer: Role of SPECT and PET in imaging bone metastases. Semin Nucl Med. 2009;39:396–407. - PubMed
-
- Breeuwsma AJ, Pruim J, Jongen MM, Suurmeijer AJ, Vaalburg W, Nijman RJ, et al. In vivo uptake of [11C] choline does not correlate with cell proliferation in human prostate cancer. Eur J Nucl Med Mol Imaging. 2005;32:668–73. - PubMed
-
- Zheng QH, Gardner TA, Raikwar S, Kao C, Stone KL, Martinez TD, et al. [11C] Choline as a PET biomarker for assessment of prostate cancer tumor models. Bioorg Med Chem. 2004;12:2887–93. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

