Effect of staurosporine in the morphology and viability of cerebellar astrocytes: role of reactive oxygen species and NADPH oxidase
- PMID: 25215174
- PMCID: PMC4151592
- DOI: 10.1155/2014/678371
Effect of staurosporine in the morphology and viability of cerebellar astrocytes: role of reactive oxygen species and NADPH oxidase
Abstract
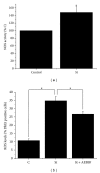
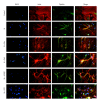
Cell death implies morphological changes that may contribute to the progression of this process. In astrocytes, the mechanisms involving the cytoskeletal changes during cell death are not well explored. Although NADPH oxidase (NOX) has been described as being a critical factor in the production of ROS, not much information is available about the participation of NOX-derived ROS in the cell death of astrocytes and their role in the alterations of the cytoskeleton during the death of astrocytes. In this study, we have evaluated the participation of ROS in the death of cultured cerebellar astrocytes using staurosporine (St) as death inductor. We found that astrocytes express NOX1, NOX2, and NOX4. Also, St induced an early ROS production and NOX activation that participate in the death of astrocytes. These findings suggest that ROS produced by St is generated through NOX1 and NOX4. Finally, we showed that the reorganization of tubulin and actin induced by St is ROS independent and that St did not change the level of expression of these cytoskeletal proteins. We conclude that ROS produced by a NOX is required for cell death in astrocytes, but not for the morphological alterations induced by St.
Figures







Similar articles
-
NADPH oxidase inhibitor VAS2870 prevents staurosporine-induced cell death in rat astrocytes.Radiol Oncol. 2019 Jan 19;53(1):69-76. doi: 10.2478/raon-2019-0002. Radiol Oncol. 2019. PMID: 30661061 Free PMC article.
-
NOX2 mediates apoptotic death induced by staurosporine but not by potassium deprivation in cerebellar granule neurons.J Neurosci Res. 2009 Aug 15;87(11):2531-40. doi: 10.1002/jnr.22079. J Neurosci Res. 2009. PMID: 19360906
-
Induction and activation by zinc of NADPH oxidase in cultured cortical neurons and astrocytes.J Neurosci. 2000 Dec 1;20(23):RC111. doi: 10.1523/JNEUROSCI.20-23-j0001.2000. J Neurosci. 2000. PMID: 11090611 Free PMC article.
-
The role of Nox-mediated oxidation in the regulation of cytoskeletal dynamics.Curr Pharm Des. 2015;21(41):6009-22. doi: 10.2174/1381612821666151029112624. Curr Pharm Des. 2015. PMID: 26510432 Free PMC article. Review.
-
The NOX toolbox: validating the role of NADPH oxidases in physiology and disease.Cell Mol Life Sci. 2012 Jul;69(14):2327-43. doi: 10.1007/s00018-012-1010-9. Epub 2012 May 31. Cell Mol Life Sci. 2012. PMID: 22648375 Free PMC article. Review.
Cited by
-
Staurosporine as a Potential Treatment for Acanthamoeba Keratitis Using Mouse Cornea as an Ex Vivo Model.Mar Drugs. 2024 Sep 18;22(9):423. doi: 10.3390/md22090423. Mar Drugs. 2024. PMID: 39330304 Free PMC article.
-
Blue light-triggered photochemistry and cytotoxicity of retinal.Cell Signal. 2020 May;69:109547. doi: 10.1016/j.cellsig.2020.109547. Epub 2020 Jan 23. Cell Signal. 2020. PMID: 31982549 Free PMC article.
-
Quantifying Cytosolic Cytochrome c Concentration Using Carbon Quantum Dots as a Powerful Method for Apoptosis Detection.Pharmaceutics. 2021 Sep 25;13(10):1556. doi: 10.3390/pharmaceutics13101556. Pharmaceutics. 2021. PMID: 34683849 Free PMC article.
-
Metabolic and Homeostatic Changes in Seizures and Acquired Epilepsy-Mitochondria, Calcium Dynamics and Reactive Oxygen Species.Int J Mol Sci. 2017 Sep 8;18(9):1935. doi: 10.3390/ijms18091935. Int J Mol Sci. 2017. PMID: 28885567 Free PMC article. Review.
-
Role of NADPH Oxidases in Blood-Brain Barrier Disruption and Ischemic Stroke.Antioxidants (Basel). 2022 Sep 30;11(10):1966. doi: 10.3390/antiox11101966. Antioxidants (Basel). 2022. PMID: 36290688 Free PMC article. Review.
References
-
- Oppenheim RW, Prevette D, Tytell M, Homma S. Naturally occurring and induced neuronal death in the chick embryo in vivo requires protein and RNA synthesis: evidence for the role of cell death genes. Developmental Biology. 1990;138(1):104–113. - PubMed
-
- Yuan J, Yankner BA. Apoptosis in the nervous system. Nature. 2000;407(6805):802–809. - PubMed
-
- Thornberry NA, Lazebnik Y. Caspases: enemies within. Science. 1998;281(5381):1312–1316. - PubMed
-
- Brewton LS, Haddad L, Azmitia EC. Colchicine-induced cytoskeletal collapse and apoptosis in N-18 neuroblastoma cultures is rapidly reversed by applied S-100β . Brain Research. 2001;912(1):9–16. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

