Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity
- PMID: 25215485
- PMCID: PMC4167398
- DOI: 10.1016/j.cell.2014.08.023
Broadly neutralizing anti-HIV-1 antibodies require Fc effector functions for in vivo activity
Abstract
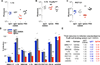
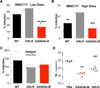
Broadly neutralizing antibodies (bNAbs) against HIV-1 provide both effective pre-exposure prophylaxis and treatment of HIV-1 infection in murine and nonhuman primate models, suggesting their potential use in humans. Although much is known about the role of variable domains in the neutralization breadth and potency of these bNAbs, the contribution of Fc domains to their activities is, by contrast, poorly characterized. Assessment of the in vivo activity of several bNAbs revealed that FcγR-mediated effector function contributes substantially to their capacity to block viral entry, suppress viremia, and confer therapeutic activity. Enhanced in vivo potency of anti-HIV-1 bNAbs was associated with preferential engagement of activating, but not inhibitory FcγRs, and Fc domain-engineered bNAb variants with selective binding capacity for activating FcγRs displayed augmented protective activity. These findings reveal key roles for Fc effector function in the in vivo activity of anti-HIV-1 bNAbs and provide strategies for generating bNAbs with improved efficacy.
Copyright © 2014 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declare no conflict of interest.
Figures


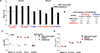

Comment in
-
HIV: Potency needs constancy.Nature. 2014 Oct 23;514(7523):442-3. doi: 10.1038/514442a. Nature. 2014. PMID: 25341782 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

