Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2
- PMID: 25215504
- PMCID: PMC4162611
- DOI: 10.1371/journal.pone.0107758
Lactobacilli inactivate Chlamydia trachomatis through lactic acid but not H2O2
Abstract
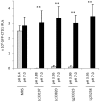
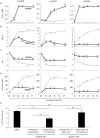
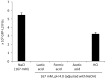
Lactobacillus species dominate the microbiome in the lower genital tract of most reproductive-age women. Producing lactic acid and H2O2, lactobacilli are believed to play an important role in prevention of colonization by and growth of pathogens. However, to date, there have been no reported studies characterizing how lactobacilli interact with Chlamydia trachomatis, a leading sexually transmitted bacterium. In this report, we demonstrate inactivation of C. trachomatis infectivity by culture media conditioned by Lactobacillus crispatus, L. gasseri and L. jensenii, known to be dominating organisms in the human vaginal microbiome. Lactobacillus still cultures produced lactic acid, leading to time- and concentration-dependent killing of C. trachomatis. Neutralization of the acidic media completely reversed chlamydia killing. Addition of lactic acid into Lactobacillus-unconditioned growth medium recapitulated the chlamydiacidal activity of conditioned media. The H2O2 concentrations in the still cultures were found to be comparable to those reported for the cervicovaginal fluid, but insufficient to inactivate chlamydiae. Aeration of Lactobacillus cultures by shaking markedly induced H2O2 production, but strongly inhibited Lactobacillus growth and lactic acid production, and thus severely affected acidification, leading to significantly reduced chlamydiacidal efficiency. These observations indicate lactobacilli inactivate chlamydiae primarily through maintaining acidity in a relatively hypoxic environment in the vaginal lumen with limited H2O2, which is consistent with the notion that women with higher vaginal pH are more prone to sexually transmitted C. trachomatis infection. In addition to lactic acid, formic acid and acetic acid also exhibited potent chlamydiacidal activities. Taken together, our findings imply that lowering the vaginal pH through engineering of the vaginal microbiome and other means will make women less susceptible to C. trachomatis infection.
Conflict of interest statement
Figures

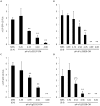
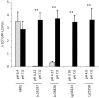
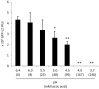
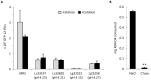

References
-
- CDC (2013) Notifiable Diseases and Mortality Tables. Morbidity and Mortality Weekly Report (MMWR) 62: 424–437. - PubMed
-
- CDC (2013) CDC fact sheet. STD trends in the United States: 2011 national data for chlamydia, gonorrhea, and syphilis
-
- Stephens RS, Myers G, Eppinger M, Bavoil PM (2009) Divergence without difference: phylogenetics and taxonomy of Chlamydia resolved. FEMS Immunol Med Microbiol 55: 115–119. - PubMed
-
- Abraham S, Toutous-Trellu L, Pechere M, Hugonnet S, Liassine N, et al. (2006) Increased incidence of sexually transmitted infections in Geneva, Switzerland. Dermatology 212: 41–46. - PubMed
-
- Vajdic CM, Middleton M, Bowden FJ, Fairley CK, Kaldor JM (2005) The prevalence of genital Chlamydia trachomatis in Australia 1997–2004: a systematic review. Sexual Health 2: 169–183. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

