Experimental validation of the role of trifluoroethanol as a nanocrowder
- PMID: 25215518
- PMCID: PMC4183368
- DOI: 10.1021/jp508056w
Experimental validation of the role of trifluoroethanol as a nanocrowder
Abstract
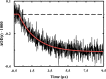
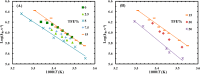
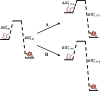
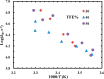
Trifluoroethanol (TFE) is commonly used to induce protein secondary structure, especially α-helix formation. Due to its amphiphilic nature, however, TFE can also self-associate to form micellelike, nanometer-sized clusters. Herein, we hypothesize that such clusters can act as nanocrowders to increase protein folding rates via the excluded volume effect. To test this hypothesis, we measure the conformational relaxation kinetics of an intrinsically disordered protein, the phosphorylated kinase inducible domain (pKID), which forms a helix-turn-helix in TFE solutions. We find that the conformational relaxation rate of pKID displays a rather complex dependence on TFE percentage (v/v): while it first decreases between 0 and 5%, between 5 and 15% the rate increases and then remains relatively unchanged between 15 and 30% and finally decreases again at higher percentages (i.e., 50%). This trend coincides with the fact that TFE clustering is maximized in the range of 15-30%, thus providing validation of our hypothesis. Another line of supporting evidence comes from the observation that the relaxation rate of a monomeric helical peptide, which due to its predominantly local interactions in the folded state is less affected by crowding, does not show a similar TFE dependence.
Figures



References
-
- Goodman M.; Listowsky I.; Masuda Y.; Boardman F. Conformational Aspects of Polypeptides. VIII. Helical Assignments via Far Ultrviolet Absorption Spectra and Optical Activity. Biopolymers 1963, 1, 33–42.
-
- Sonnichsen F. D.; Van Eyk J. E.; Hodges R. S.; Sykes B. D. Effect of Trifluoroethanol on Protein Secondary Structure: An NMR and CD Study Using a Synthetic Actin Peptide. Biochemistry 1992, 31, 8790–8798. - PubMed
-
- Buck M. Trifluoroethanol and Colleagues: Cosolvents Come of Age. Recent Studies with Peptides and Proteins. Q. Rev. Biophys. 1998, 31, 297–355. - PubMed
-
- Rajan R.; Balaram P. A Model for the Interaction of Trifluoroethanol with Peptides and Proteins. Int. J. Pept. Protein Res. 1996, 48, 328–336. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

