Understanding the interaction determinants of CAPN1 inhibition by CAST4 from bovines using molecular modeling techniques
- PMID: 25215589
- PMCID: PMC6271145
- DOI: 10.3390/molecules190914316
Understanding the interaction determinants of CAPN1 inhibition by CAST4 from bovines using molecular modeling techniques
Abstract
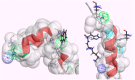
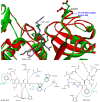
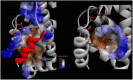
HCV-induced CAPN activation and its effects on virus-infected cells in a host-immune system have been studied recently. It has been shown that the HCV-nonstructural 5A protein acts as both an inducer and a substrate for host CAPN protease; it participates in suppressing the TNF-α-induced apoptosis response and downstream IFN-induced antiviral processes. However, little is known regarding the disturbance of antiviral responses generated by bovine CAPN activation by BVDV, which is a surrogate model of HCV and is one of the most destructive diseases leading to great economic losses in cattle herds worldwide. This is also thought to be associated with the effects of either small CAPN inhibitors or the natural inhibitor CAST. They mainly bind to the binding site of CAPN substrate proteins and competitively inhibit the binding of the enzyme substrates to possibly defend against the two viruses (HCV and BVDV) for anti-viral immunity. To devise a new stratagem to discover lead candidates for an anti-BVDV drug, we first attempted to understand the bovine CAPN-CAST interaction sites and the interaction constraints of local binding architectures, were well reflected in the geometry between the pharmacophore features and its shape constraints identified using our modeled bovine CAPN1/CAST4 complex structures. We propose a computer-aided molecular design of an anti-BVDV drug as a mimetic CAST inhibitor to develop a rule-based screening function for adjusting the puzzle of relationship between bovine CAPN1 and the BVDV nonstructural proteins from all of the data obtained in the study.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Homology modeling study of bovine μ-calpain inhibitor-binding domains.Int J Mol Sci. 2014 May 6;15(5):7897-938. doi: 10.3390/ijms15057897. Int J Mol Sci. 2014. PMID: 24806345 Free PMC article.
-
Downregulation of the Long Noncoding RNA IALNCR Targeting MAPK8/JNK1 Promotes Apoptosis and Antagonizes Bovine Viral Diarrhea Virus Replication in Host Cells.J Virol. 2022 Sep 14;96(17):e0111322. doi: 10.1128/jvi.01113-22. Epub 2022 Aug 22. J Virol. 2022. PMID: 35993735 Free PMC article.
-
Establishment of a subgenomic replicon for bovine viral diarrhea virus in Huh-7 cells and modulation of interferon-regulated factor 3-mediated antiviral response.J Virol. 2005 Mar;79(5):2788-96. doi: 10.1128/JVI.79.5.2788-2796.2005. J Virol. 2005. PMID: 15708997 Free PMC article.
-
What is known about the antiviral agents active against bovine viral diarrhea virus (BVDV)?Curr Med Chem. 2010;17(26):2933-55. doi: 10.2174/092986710792065036. Curr Med Chem. 2010. PMID: 20858174 Review.
-
The persistence of bovine viral diarrhea virus.Biologicals. 2003 Jun;31(2):133-5. doi: 10.1016/s1045-1056(03)00029-0. Biologicals. 2003. PMID: 12770545 Review.
Cited by
-
Experimental Verification of CAPN1 and CAST Gene Polymorphisms in Different Generations of Da-Heng Broilers.Biomed Res Int. 2017;2017:7968450. doi: 10.1155/2017/7968450. Epub 2017 Jun 21. Biomed Res Int. 2017. PMID: 28713829 Free PMC article.
-
In silico affinity profiling of neuroactive polyphenols for post-traumatic calpain inactivation: a molecular docking and atomistic simulation sensitivity analysis.Molecules. 2014 Dec 23;20(1):135-68. doi: 10.3390/molecules20010135. Molecules. 2014. PMID: 25546626 Free PMC article.
References
-
- Croall A.D., DeMartino G.N. Calcium-activated neutral protease (calpain) systems structure, function, and regulation. Physiol. Rev. 1991;71:813–847. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

