Forced IDO1 expression in dendritic cells restores immunoregulatory signalling in autoimmune diabetes
- PMID: 25215657
- PMCID: PMC4193887
- DOI: 10.1111/jcmm.12360
Forced IDO1 expression in dendritic cells restores immunoregulatory signalling in autoimmune diabetes
Abstract
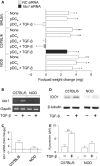
Indoleamine 2,3-dioxygenase (IDO1), a tryptophan catabolizing enzyme, is recognized as an authentic regulator of immunity in several physiopathologic conditions. We have recently demonstrated that IDO1 does not merely degrade tryptophan and produce immunoregulatory kynurenines, but it also acts as a signal-transducing molecule, independently of its enzymic function. IDO1 signalling activity is triggered in plasmacytoid dendritic cells (pDCs) by transforming growth factor-β (TGF-β), an event that requires the non-canonical NF-κB pathway and induces long-lasting IDO1 expression and autocrine TGF-β production in a positive feedback loop, thus sustaining a stably regulatory phenotype in pDCs. IDO1 expression and catalytic function are defective in pDCs from non-obese diabetic (NOD) mice, a prototypic model of autoimmune diabetes. In the present study, we found that TGF-β failed to activate IDO1 signalling function as well as up-regulate IDO1 expression in NOD pDCs. Moreover, TGF-β-treated pDCs failed to exert immunosuppressive properties in vivo. Nevertheless, transfection of NOD pDCs with Ido1 prior to TGF-β treatment resulted in activation of the Ido1 promoter and induction of non-canonical NF-κB and TGF-β, as well as decreased production of the pro-inflammatory cytokines, interleukin 6 (IL-6) and tumour necrosis factor-α (TNF-α). Overexpression of IDO1 in TGF-β-treated NOD pDCs also resulted in pDC ability to suppress the in vivo presentation of a pancreatic β-cell auto-antigen. Thus, our data suggest that a correction of IDO1 expression may restore its dual function and thus represent a proper therapeutic manoeuvre in this autoimmune setting.
Keywords: IDO1; autoimmune diabetes; immune regulation; non-canonical NF-κB; non-obese diabetic (NOD) mice; plasmacytoid dendritic cells; tryptophan catabolism.
© 2014 The Authors. Journal of Cellular and Molecular Medicine published by John Wiley & Sons Ltd and Foundation for Cellular and Molecular Medicine.
Figures



References
-
- Kishimoto H, Sprent J. A defect in central tolerance in NOD mice. Nat Immunol. 2001;2:1025–31. - PubMed
-
- Liu J, Beller D. Aberrant production of IL-12 by macrophages from several autoimmune-prone mouse strains is characterized by intrinsic and unique patterns of NF-κB expression and binding to the IL-12 p40 promoter. Immunol. 2002;169:581–6. - PubMed
-
- Suarez-Pinzon WL, Mabley JG, Strynadka K, et al. An inhibitor of inducible nitric oxide synthase and scavenger of peroxynitrite prevents diabetes development in NOD mice. J Autoimmun. 2001;16:449–55. - PubMed
-
- Serreze DV, Gaskins HR, Leiter EH. Defects in the differentiation and function of antigen presenting cells in NOD/Lt mice. J Immunol. 1993;150:2534–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

