Novel mucosal DNA-MVA HIV vaccination in which DNA-IL-12 plus cholera toxin B subunit (CTB) cooperates to enhance cellular systemic and mucosal genital tract immunity
- PMID: 25215887
- PMCID: PMC4162600
- DOI: 10.1371/journal.pone.0107524
Novel mucosal DNA-MVA HIV vaccination in which DNA-IL-12 plus cholera toxin B subunit (CTB) cooperates to enhance cellular systemic and mucosal genital tract immunity
Abstract
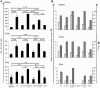
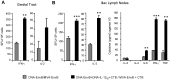
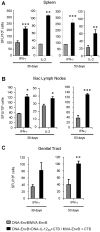
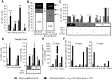
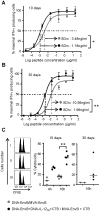
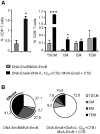
Induction of local antiviral immune responses at the mucosal portal surfaces where HIV-1 and other viral pathogens are usually first encountered remains a primary goal for most vaccines against mucosally acquired viral infections. Exploring mucosal immunization regimes in order to find optimal vector combinations and also appropriate mucosal adjuvants in the HIV vaccine development is decisive. In this study we analyzed the interaction of DNA-IL-12 and cholera toxin B subunit (CTB) after their mucosal administration in DNA prime/MVA boost intranasal regimes, defining the cooperation of both adjuvants to enhance immune responses against the HIV-1 Env antigen. Our results demonstrated that nasal mucosal DNA/MVA immunization schemes can be effectively improved by the co-delivery of DNA-IL-12 plus CTB inducing elevated HIV-specific CD8 responses in spleen and more importantly in genital tract and genito-rectal draining lymph nodes. Remarkably, these CTL responses were of superior quality showing higher avidity, polyfunctionality and a broader cytokine profile. After IL-12+CTB co-delivery, the cellular responses induced showed an enhanced breadth recognizing with higher efficiency Env peptides from different subtypes. Even more, an in vivo CTL cytolytic assay demonstrated the higher specific CD8 T-cell performance after the IL-12+CTB immunization showing in an indirect manner its potential protective capacity. Improvements observed were maintained during the memory phase where we found higher proportions of specific central memory and T memory stem-like cells T-cell subpopulations. Together, our data show that DNA-IL-12 plus CTB can be effectively employed acting as mucosal adjuvants during DNA prime/MVA boost intranasal vaccinations, enhancing magnitude and quality of HIV-specific systemic and mucosal immune responses.
Conflict of interest statement
Figures

Similar articles
-
Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule.J Immunol. 2004 May 15;172(10):6209-20. doi: 10.4049/jimmunol.172.10.6209. J Immunol. 2004. PMID: 15128809
-
Improving recombinant MVA immune responses: potentiation of the immune responses to HIV-1 with MVA and DNA vectors expressing Env and the cytokines IL-12 and IFN-gamma.Virus Res. 2006 Mar;116(1-2):11-20. doi: 10.1016/j.virusres.2005.08.008. Epub 2005 Oct 7. Virus Res. 2006. PMID: 16214252
-
DNA-MVA-protein vaccination of rhesus macaques induces HIV-specific immunity in mucosal-associated lymph nodes and functional antibodies.Vaccine. 2017 Feb 7;35(6):929-937. doi: 10.1016/j.vaccine.2016.12.060. Epub 2017 Jan 6. Vaccine. 2017. PMID: 28069361 Free PMC article.
-
Mucosal HIV vaccines: where are we now?Curr HIV Res. 2004 Jan;2(1):1-10. doi: 10.2174/1570162043485004. Curr HIV Res. 2004. PMID: 15053336 Review.
-
Towards a new generation of vaccines: the cytokine IL-12 as an adjuvant to enhance cellular immune responses to pathogens during prime-booster vaccination regimens.Histol Histopathol. 2001 Apr;16(2):655-67. doi: 10.14670/HH-16.655. Histol Histopathol. 2001. PMID: 11332721 Review.
Cited by
-
Development of Nasal Vaccines and the Associated Challenges.Pharmaceutics. 2022 Sep 20;14(10):1983. doi: 10.3390/pharmaceutics14101983. Pharmaceutics. 2022. PMID: 36297419 Free PMC article. Review.
-
Localized and Systemic Immune Responses against SARS-CoV-2 Following Mucosal Immunization.Vaccines (Basel). 2021 Feb 6;9(2):132. doi: 10.3390/vaccines9020132. Vaccines (Basel). 2021. PMID: 33562141 Free PMC article.
-
Advances in Infectious Disease Vaccine Adjuvants.Vaccines (Basel). 2022 Jul 13;10(7):1120. doi: 10.3390/vaccines10071120. Vaccines (Basel). 2022. PMID: 35891284 Free PMC article. Review.
-
Multiplexed Fc array for evaluation of antigen-specific antibody effector profiles.J Immunol Methods. 2017 Apr;443:33-44. doi: 10.1016/j.jim.2017.01.010. Epub 2017 Feb 3. J Immunol Methods. 2017. PMID: 28163018 Free PMC article.
-
Cholera toxin B: one subunit with many pharmaceutical applications.Toxins (Basel). 2015 Mar 20;7(3):974-96. doi: 10.3390/toxins7030974. Toxins (Basel). 2015. PMID: 25802972 Free PMC article. Review.
References
-
- Haase AT (2005) Perils at mucosal front lines for HIV and SIV and their hosts. Nat Rev Immunol 5: 783–792. - PubMed
-
- Haase AT (2010) Targeting early infection to prevent HIV-1 mucosal transmission. Nature 464: 217–223. - PubMed
-
- Cripps AW, Kyd JM, Foxwell AR (2001) Vaccines and mucosal immunisation. Vaccine 19: 2513–2515. - PubMed
-
- Gherardi MM, Perez-Jimenez E, Najera JL, Esteban M (2004) Induction of HIV immunity in the genital tract after intranasal delivery of a MVA vector: enhanced immunogenicity after DNA prime-modified vaccinia virus Ankara boost immunization schedule. J Immunol 172: 6209–6220. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

