Autoreactivity to glucose regulated protein 78 links emphysema and osteoporosis in smokers
- PMID: 25216103
- PMCID: PMC4162538
- DOI: 10.1371/journal.pone.0105066
Autoreactivity to glucose regulated protein 78 links emphysema and osteoporosis in smokers
Abstract
Rationale: Emphysema and osteoporosis are epidemiologically associated diseases of cigarette smokers. The causal mechanism(s) linking these illnesses is unknown. We hypothesized autoimmune responses may be involved in both disorders.
Objectives: To discover an antigen-specific autoimmune response associated with both emphysema and osteoporosis among smokers.
Methods: Replicate nonbiased discovery assays indicated that autoimmunity to glucose regulated protein 78 (GRP78), an endoplasmic reticulum chaperone and cell surface signaling receptor, is present in many smokers. Subject assessments included spirometry, chest CT scans, dual x-ray absorptiometry, and immunoblots for anti-GRP78 IgG. Anti-GRP78 autoantibodies were isolated from patient plasma by affinity chromatography, leukocyte functions assessed by flow cytometry, and soluble metabolites and mediators measured by immunoassays.
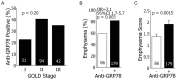
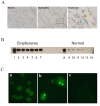
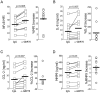
Measurements and main results: Circulating anti-GRP78 IgG autoantibodies were detected in plasma specimens from 86 (32%) of the 265 smoking subjects. Anti-GRP78 autoantibodies were singularly prevalent among subjects with radiographic emphysema (OR 3.1, 95%CI 1.7-5.7, p = 0.003). Anti-GRP78 autoantibodies were also associated with osteoporosis (OR 4.7, 95%CI 1.7-13.3, p = 0.002), and increased circulating bone metabolites (p = 0.006). Among emphysematous subjects, GRP78 protein was an autoantigen of CD4 T-cells, stimulating lymphocyte proliferation (p = 0.0002) and IFN-gamma production (p = 0.03). Patient-derived anti-GRP78 autoantibodies had avidities for osteoclasts and macrophages, and increased macrophage NFkB phosphorylation (p = 0.005) and productions of IL-8, CCL-2, and MMP9 (p = 0.005, 0.007, 0.03, respectively).
Conclusions: Humoral and cellular GRP78 autoimmune responses in smokers have numerous biologically-relevant pro-inflammatory and other deleterious actions, and are associated with emphysema and osteoporosis. These findings may have relevance for the pathogenesis of smoking-associated diseases, and development of biomarker immunoassays and/or novel treatments for these disorders.
Conflict of interest statement
Figures


Similar articles
-
Radiographic Emphysema, Circulating Bone Biomarkers, and Progressive Bone Mineral Density Loss in Smokers.Ann Am Thorac Soc. 2018 May;15(5):615-621. doi: 10.1513/AnnalsATS.201709-743OC. Ann Am Thorac Soc. 2018. PMID: 29328885 Free PMC article.
-
Glucose-Regulated Protein 78 Autoantibodies Are Associated with Carotid Atherosclerosis in Chronic Obstructive Pulmonary Disease Patients.Immunohorizons. 2020 Feb 21;4(2):108-118. doi: 10.4049/immunohorizons.1900098. Immunohorizons. 2020. PMID: 32086320 Free PMC article.
-
Reduced Bone Density and Vertebral Fractures in Smokers. Men and COPD Patients at Increased Risk.Ann Am Thorac Soc. 2015 May;12(5):648-56. doi: 10.1513/AnnalsATS.201412-591OC. Ann Am Thorac Soc. 2015. PMID: 25719895 Free PMC article.
-
Glucose-regulated protein (GRP78) is an important cell surface receptor for viral invasion, cancers, and neurological disorders.IUBMB Life. 2021 Jun;73(6):843-854. doi: 10.1002/iub.2502. Epub 2021 May 15. IUBMB Life. 2021. PMID: 33960608 Review.
-
Does radiographic emphysema correlate with low bone mineral density?Curr Opin Pulm Med. 2012 Mar;18(2):125-30. doi: 10.1097/MCP.0b013e32834f8194. Curr Opin Pulm Med. 2012. PMID: 22273685 Review.
Cited by
-
Contribution of adaptive immunity to human COPD and experimental models of emphysema.Physiol Rev. 2023 Apr 1;103(2):1059-1093. doi: 10.1152/physrev.00036.2021. Epub 2022 Oct 6. Physiol Rev. 2023. PMID: 36201635 Free PMC article. Review.
-
Bisphosphonates Reduce Smoking-Induced Osteoporotic-Like Alterations by Regulating RANKL/OPG in an Osteoblast and Osteoclast Co-Culture Model.Int J Mol Sci. 2020 Dec 23;22(1):53. doi: 10.3390/ijms22010053. Int J Mol Sci. 2020. PMID: 33374546 Free PMC article.
-
Endoplasmic reticulum stress and glutathione therapeutics in chronic lung diseases.Redox Biol. 2020 Jun;33:101516. doi: 10.1016/j.redox.2020.101516. Epub 2020 Mar 23. Redox Biol. 2020. PMID: 32249209 Free PMC article. Review. No abstract available.
-
A Phenome-Wide Association Study Uncovers a Role for Autoimmunity in the Development of Chronic Obstructive Pulmonary Disease.Am J Respir Cell Mol Biol. 2018 Jun;58(6):777-779. doi: 10.1165/rcmb.2017-0409LE. Am J Respir Cell Mol Biol. 2018. PMID: 29856256 Free PMC article. No abstract available.
-
Is osteoporosis an autoimmune mediated disorder?Bone Rep. 2017 Oct 16;7:121-131. doi: 10.1016/j.bonr.2017.10.003. eCollection 2017 Dec. Bone Rep. 2017. PMID: 29124082 Free PMC article. Review.
References
-
- Mannino DM (2002) COPD: epidemiology, prevalence, morbidity and mortality, and disease heterogeneity. Chest 121: 121S–26S. - PubMed
-
- Washko GR, Criner GJ, Mohsenifar Z, Sciurba FC, Sharafkhaneh A, et al. (2008) Computed tomographic-based quantification of emphysema and correlation to pulmonary function and mechanics. COPD 5: 177–86. - PubMed
-
- Sin DD, Man SF (2005) Chronic obstructive pulmonary disease as a risk factor for cardiovascular morbidity and mortality. Proc Amer Thorac Soc 2: 8–11. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

