Dynamics of chronic myeloid leukemia response to dasatinib, nilotinib, and high-dose imatinib
- PMID: 25216683
- PMCID: PMC4222479
- DOI: 10.3324/haematol.2013.085977
Dynamics of chronic myeloid leukemia response to dasatinib, nilotinib, and high-dose imatinib
Abstract
Treatment with the tyrosine kinase inhibitor imatinib is the standard of care for newly diagnosed patients with chronic myeloid leukemia. In recent years, several second-generation inhibitors - such as dasatinib and nilotinib - have become available: these promise to overcome some of the mutations associated with acquired resistance to imatinib. Despite eliciting similar clinical responses, the molecular effects of these agents on different subpopulations of leukemic cells remain incompletely understood. Furthermore, the consequences of using high-dose imatinib therapy have not been investigated in detail. Here we utilized clinical data from patients treated with dasatinib, nilotinib, or high-dose imatinib, together with a statistical data analysis and mathematical modeling approach, to investigate the molecular treatment response of leukemic cells to these agents. We found that these drugs elicit very similar responses if administered front-line. However, patients display significantly different kinetics when treated second-line, both in terms of differences between front-line and second-line treatment for the same drug, and among agents when used as second-line. We then utilized a mathematical framework describing the behavior of four differentiation levels of leukemic cells during therapy to predict the treatment response kinetics for the different cohorts of patients. The dynamics of BCR-ABL1 clearance observed in our study suggest that the use of standard or high-dose imatinib or a second-generation tyrosine kinase inhibitor such as nilotinib or dasatinib elicits similar responses when administered as front-line therapy for patients with chronic myeloid leukemia in chronic phase.
Copyright© Ferrata Storti Foundation.
Figures
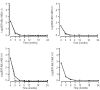

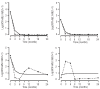
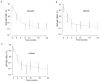
References
-
- Druker BJ, Tamura S, Buchdunger E, Ohno S, Segal GM, Fanning S, et al. Effects of a selective inhibitor of the Abl tyrosine kinase on the growth of Bcr-Abl positive cells. Nat Med. 1996;2(5):561–6. - PubMed
-
- Druker BJ, Talpaz M, Resta DJ, Peng B, Buchdunger E, Ford JM, et al. Efficacy and safety of a specific inhibitor of the BCR-ABL tyrosine kinase in chronic myeloid leukemia. N Engl J Med. 2001;344(14): 1031–7. - PubMed
-
- Kantarjian H, Sawyers C, Hochhaus A, Guilhot F, Schiffer C, Gambacorti-Passerini C, et al. Hematologic and cytogenetic responses to imatinib mesylate in chronic myelogenous leukemia. N Engl J Med. 2002;346(9):645–52. - PubMed
-
- Gorre ME, Mohammed M, Ellwood K, Hsu N, Paquette R, Rao PN, et al. Clinical resistance to STI-571 cancer therapy caused by BCR-ABL gene mutation or amplification. Science. 2001;293(5531):876–80. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

