CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice
- PMID: 25217165
- PMCID: PMC4185219
- DOI: 10.4049/jimmunol.1401597
CD8+ T cells prevent antigen-induced antibody-dependent enhancement of dengue disease in mice
Abstract
Dengue virus (DENV) causes pathologies ranging from the febrile illness dengue fever to the potentially lethal severe dengue disease. A major risk factor for developing severe dengue disease is the presence of subprotective DENV-reactive Abs from a previous infection (or from an immune mother), which can induce Ab-dependent enhancement of infection (ADE). However, infection in the presence of subprotective anti-DENV Abs does not always result in severe disease, suggesting that other factors influence disease severity. In this study we investigated how CD8(+) T cell responses influence the outcome of Ab-mediated severe dengue disease. Mice were primed with aluminum hydroxide-adjuvanted UV-inactivated DENV prior to challenge with DENV. Priming failed to induce robust CD8(+) T cell responses, and it induced nonneutralizing Ab responses that increased disease severity upon infection. Transfer of exogenous DENV-activated CD8(+) T cells into primed mice prior to infection prevented Ab-dependent enhancement and dramatically reduced viral load. Our results suggest that in the presence of subprotective anti-DENV Abs, efficient CD8(+) T cell responses reduce the risk of Ab-mediated severe dengue disease.
Copyright © 2014 by The American Association of Immunologists, Inc.
Figures

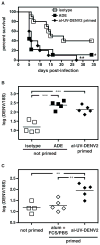



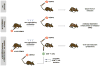
Similar articles
-
Maternally Acquired Zika Antibodies Enhance Dengue Disease Severity in Mice.Cell Host Microbe. 2018 Nov 14;24(5):743-750.e5. doi: 10.1016/j.chom.2018.09.015. Cell Host Microbe. 2018. PMID: 30439343 Free PMC article.
-
Dengue Virus Infection with Highly Neutralizing Levels of Cross-Reactive Antibodies Causes Acute Lethal Small Intestinal Pathology without a High Level of Viremia in Mice.J Virol. 2015 Jun;89(11):5847-61. doi: 10.1128/JVI.00216-15. Epub 2015 Mar 18. J Virol. 2015. PMID: 25787279 Free PMC article.
-
A Dengue Virus Serotype 1 mRNA-LNP Vaccine Elicits Protective Immune Responses.J Virol. 2021 May 24;95(12):e02482-20. doi: 10.1128/JVI.02482-20. Print 2021 May 24. J Virol. 2021. PMID: 33762420 Free PMC article.
-
Modulation of Dengue/Zika Virus Pathogenicity by Antibody-Dependent Enhancement and Strategies to Protect Against Enhancement in Zika Virus Infection.Front Immunol. 2018 Apr 23;9:597. doi: 10.3389/fimmu.2018.00597. eCollection 2018. Front Immunol. 2018. PMID: 29740424 Free PMC article. Review.
-
Antibody-Dependent Enhancement: A Challenge for Developing a Safe Dengue Vaccine.Front Cell Infect Microbiol. 2020 Oct 22;10:572681. doi: 10.3389/fcimb.2020.572681. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 33194810 Free PMC article. Review.
Cited by
-
Can Complementary Prime-Boost Immunization Strategies Be an Alternative and Promising Vaccine Approach Against Dengue Virus?Front Immunol. 2019 Aug 27;10:1956. doi: 10.3389/fimmu.2019.01956. eCollection 2019. Front Immunol. 2019. PMID: 31507591 Free PMC article. Review.
-
Zika Virus-Immune Plasmas from Symptomatic and Asymptomatic Individuals Enhance Zika Pathogenesis in Adult and Pregnant Mice.mBio. 2019 Jul 2;10(4):e00758-19. doi: 10.1128/mBio.00758-19. mBio. 2019. PMID: 31266863 Free PMC article.
-
Immune Responses to Dengue and Zika Viruses-Guidance for T Cell Vaccine Development.Int J Environ Res Public Health. 2018 Feb 23;15(2):385. doi: 10.3390/ijerph15020385. Int J Environ Res Public Health. 2018. PMID: 29473899 Free PMC article. Review.
-
Cross-Protection against MERS-CoV by Prime-Boost Vaccination Using Viral Spike DNA and Protein.J Virol. 2020 Nov 23;94(24):e01176-20. doi: 10.1128/JVI.01176-20. Print 2020 Nov 23. J Virol. 2020. PMID: 32967955 Free PMC article.
-
Peptide-Based Subunit Vaccine Design of T- and B-Cells Multi-Epitopes against Zika Virus Using Immunoinformatics Approaches.Microorganisms. 2019 Jul 31;7(8):226. doi: 10.3390/microorganisms7080226. Microorganisms. 2019. PMID: 31370224 Free PMC article.
References
-
- Guzman MG, Alvarez M, Halstead SB. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: an historical perspective and role of antibody-dependent enhancement of infection. Archives of virology. 2013;158:1445–1459. - PubMed
-
- Carod-Artal FJ, Wichmann O, Farrar J, Gascon J. Neurological complications of dengue virus infection. Lancet neurology. 2013;12:906–919. - PubMed
-
- Halstead SB. Dengue. Lancet. 2007;370:1644–1652. - PubMed
-
- World Health Organization. Dengue guidelines for diagnosis, treatment, prevention and control. WHO Press; Geneva: 2009. New edition. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

